Summary:
Objective:
We assessed body composition, bone mineral density (BMD), glucose, and lipids in Williams syndrome (WS), a rare microdeletion disorder.
Design:
Individuals with WS had outpatient assessment at Massachusetts General Hospital. Controls were selected from the National Health and Nutrition Examination Survey (NHANES 2005–2006).
Patients:
22 individuals with WS, each matched by age, sex, and race to 4 NHANES controls
Measurements:
Blood sampling, oral glucose tolerance test, dual energy x-ray absorptiometry (DXA) scan
Results:
WS and control groups were 59% female and 29 ± 8 years old. Compared to controls, individuals with WS were shorter but had similar body weight, with more fat and less lean mass. Percent body fat was higher in WS even after adjusting for BMI (+2.1% [95% CI 0.4, 3.9%]). Four WS patients had abnormal lower extremity fat accumulation resembling lipedema. HbA1c (+0.5% [0.2, 0.7]) and 2-hour glucose (+68mg/dL [44, 93]) were higher in WS versus controls, differences which persisted after adjusting for BMI. Fasting glucose was comparable between groups. LDL (−18mg/dL [−35, −2]) and triglycerides (−45mg/dL [−87, −2]) were significantly lower in WS. Whole body BMD was significantly lower (−0.15g/cm2 [−0.20, −0.11]) in WS, and this remained true controlling for height (−0.06 g/cm2 [−0.11, −0.02]). Vitamin D was <30ng/mL in 81% of those with WS.
Conclusions:
On average, adults with WS have increased fat, decreased lean mass, impaired glucose homeostasis, and reduced BMD. Clinical efforts to build muscle and bone mass, and to ensure vitamin D sufficiency, are warranted. Genotype-phenotype research efforts are also warranted.
Keywords: Williams syndrome, bone mineral density, glucose, lipids, lean mass, fat mass, energy expenditure
Introduction
Williams syndrome (WS) is a micro-deletion disorder involving loss of a contiguous stretch of 26–28 genes on chromosome 7q11.23. It is characterized by vascular stenoses, a distinctive pattern of neurodevelopmental findings, and several endocrine and metabolic abnormalities, including impaired glucose tolerance (IGT) and abnormal body composition. Previous work has shown that up to 75% of adults with WS have IGT or diabetes on oral glucose tolerance testing (OGTT), and also that bone mineral density is decreased in WS (1, 2). Moreover, clinical observations and preliminary data suggest increased overall body fat in adults with WS, as well as a relative increase in fat deposition in the lower extremities (2). Genes in the deleted region include candidate genes that might underlie these abnormalities, such as syntaxin 1A (STX-1A) and carbohydrate-responsive element-binding protein (ChREBP), both of which are known to affect glucose and lipid metabolism (3, 4), and LIM kinase 1 (LIMK1) and frizzled-9 (FZD9), both of which affect bone mass in animal models (5).
Although there is evidence that adults with WS have metabolic perturbations and abnormal body composition, these parameters remain incompletely characterized. The purpose of this study was to more comprehensively investigate body composition, bone density, metabolic parameters, and interrelationships between these variables, in adults with WS. We demonstrate that individuals with WS, on average, have reduced lean mass, increased fat mass, decreased bone mineral density, and impaired glucose homeostasis. These findings have significant implications for clinical recommendations in this population.
Methods
Twenty-two men and women with WS participated in the study, conducted at the Massachusetts General Hospital (MGH) Clinical Research Center (CRC). Eligibility criteria were as follows: age 14–70 years; no history of weight loss surgery, liposuction, or current use of weight-lowering drugs; absence of another medical condition besides WS (e.g., Cushing syndrome) known to cause abnormal fat distribution; and negative urine pregnancy test. The diagnosis of WS was confirmed by genetic testing (either FISH or chromosomal microarray) in 21/22 subjects; the diagnosis was clinically established by an experienced examiner (BRP) in the remaining subject. Participants arrived in the morning after an 8-hour fast and underwent the following: blood sampling and indirect calorimetry in the fasting state; oral glucose tolerance test (OGTT) with consumption of a 75g glucose beverage and blood sampling at 30, 60, 90, and 120 minutes after consumption; dual x-ray absorptiometry (DXA) scanning (Hologic Discovery A); anthropometric measurements in triplicate by trained CRC bionutrition staff; and completion of a detailed medical history questionnaire. Three participants known to have diabetes prior to study entry did not undergo OGTT. Venous access for phlebotomy could not be achieved for one subject with known diabetes, and sufficient venous access for an OGTT could not be obtained for one subject without known diabetes. Expected basal metabolic rate was calculated using the Harris-Benedict equation (6). Indirect calorimetry data for one subject were discarded due to an implausible respiratory quotient of 1.5. The study was approved by the Partners HealthCare Institutional Review Board, and all subjects as well as a guardian provided informed consent for participation.
Assays performed included standard measurement of complete blood count, electrolytes, glucose, lipid panel, HbA1c, free thyroxine, and liver function tests (Laboratory Corporation of America [LabCorp], Raritan, NJ), as well as thyroid stimulating hormone [TSH] (MGH core laboratory). Serum was stored at −80C until assay for parathyroid hormone (PTH), intact aminoterminal propeptide of type I procollagen (PINP), c-terminal telopeptide (CTX), bone specific alkaline phosphatase (BSAP), 25-hydroxyvitamin D (25OHD), total adiponectin, estradiol and estrone, total testosterone, sex hormone binding globulin (SHBG), and dehydroepiandrosterone sulfate (DHEA-S). Performance details for these assays are provided in the Supplemental Information.
NHANES Controls
National Health and Nutrition Examination Survey data from 2005–2006 were used to find matched controls for each subject with WS. One-to-four matching was performed using controls randomly selected from the 2005–2006 dataset and matched on sex, race, and age (within 2 years). Matching was performed by adopting a published SAS Macro (7). Whole body DXA scans in NHANES 2005–2006 were performed using Hologic QDR-4500A fan-beam densitometer (Hologic, Inc., Bedford, Ma). When available, laboratory results for NHANES participants were compared to those for WS participants, although it is important to note that different laboratories performed the assays for the NHANES controls. Assay information for PTH and 25OHD is in the Supplemental Information.
Statistical Analysis
SAS 9.4 (SAS Institute Inc., NC) was used. To account for matching, all analyses comparing WS to NHANES controls used mixed effects multivariable linear regression (PROC MIXED) in which matched pair was treated as a random effect. To explore differences in physiologic relationships between WS and controls, multivariable linear regression (PROC GLM) with interaction terms was utilized. Unless otherwise specified, data are presented as mean ± standard deviation, or effect size [95% confidence interval (CI)].
Results
This study included 22 individuals with Williams syndrome (WS), 9 males and 13 females, with age range 16.5–48.3y. Data from 88 age-, sex- and race-matched individuals from NHANES were used as a control group. Per design, there were no between-group differences in age (28.5±7.9y in WS, 28.5±7.8y in Controls), sex (59.1% female in both groups), or race (90.9% white and 9.1% “other race” in both groups). The WS subjects were substantially shorter than the NHANES controls (155.0±6.5 vs. 169.6±9.2cm, WS vs. Controls, p<0.0001), whereas body weight was similar (72.0±22.5 vs. 71.7±15.9kg, WS vs. Controls, p=0.92). Thus, the NHANES controls had substantially lower body mass index (BMI, 24.7±4.3 kg/m2 for Controls vs. 30.0 ± 9.3 kg/m2 for WS, p=0.0002).
Body Composition
Compared to controls, individuals with WS had more fat mass and less lean mass (Table 1). Percent body fat was higher in WS (+7.1% [95% CI 4.1, 10.0]), and this difference was attenuated but remained significant even after adjusting for BMI (+2.1% [95% CI 0.4, 3.9]). Both fat mass and lean mass increased with increasing BMI, but lean mass accrual was attenuated in WS. Compared to NHANES controls, individuals with WS had the same increase in fat mass for every 1kg/m2 increase in BMI (Figure 1A), but had 0.85 kg/m2 (95% CI 0.23, 1.47kg/m2) less increase in lean mass for every 1kg/m2 increase in BMI (Figure 1B). Modeling of lean mass as predicted by BMI, WS, and an interaction between WS and BMI indicated that controls had an increase of 1.6kg in lean mass for every 1kg/m2 increase in BMI, whereas individuals with WS had an increase of only 0.75kg in lean mass for every 1kg/m2 increase in BMI (p-value for interaction between WS and BMI = 0.008). In contrast, accrual of fat mass with increasing BMI did not differ by WS status (p-value for interaction between WS and BMI = 0.78).
Table 1:
Body Composition, Glucose and Lipids, and Bone Density in WS and Controls
| Williams Syndrome (N=22) |
NHANES Matched Controls (N=88) |
Effect Size (95% CI), WS compared to Controls | ||
|---|---|---|---|---|
| Unadjusted | Adjusted for BMI | |||
| Body Composition | ||||
| Total fat (kg) | 29.2 ± 15.4 | 22.3 ± 8.3 | 6.8 (2.3, 11.4)* | −1.8 (−3.3, −0.3)* |
| Total lean (kg) | 42.1 ± 9.9 | 47.7 ± 11.6 | −5.6 (−9.3, −1.8)* | −11.3 (−13.9, −8.7)* |
| Percent Fat (%) | 37.7 ± 11.6 | 30.6 ± 8.0 | 7.1 (4.1, 10.0)* | 2.1 (0.4, 3.9)* |
| Android:Gynoid Ratio | 0.94 ± 0.14 | 0.86 0.19 | 0.08 (0.01, 0.16)* | −0.00 (−0.07, 0.07) |
| Trunk to Limb Fat Ratio | 0.87 ± 0.15 | 0.87 ± 0.22 | 0.00 (−0.07, 0.08) | −0.05 (−0.13, 0.03) |
| Glycemic Parameters | ||||
| HbA1c (%) | 5.6 ± 0.3 | 5.1 ± 0.6 | 0.5 (0.2, 0.7)* | 0.4 (0.1, 0.7)* |
| 2 hour glucose (mg/dL)a | 164 ± 56 | 96 ± 32 | 68 (44, 93)* | 66 (39, 92)* |
| Fasting glucose (mg/dL) | 90 ± 7 | 88 ± 16 | 3 (−4, 10) | 1 (−6, 8) |
| Lipids | ||||
| Total cholesterol (mg/dL) | 167 ± 29 | 190 ± 38 | −22 (−40, −5)* | −27 (−45, −8)* |
| HDL (mg/dL) | 57 ± 14 | 57 ± 15 | −0.4 (−7.5, 6.7) | 4 (−4, 11) |
| LDL (mg/dL) | 95 ± 25 | 114 ± 33 | −18 (−35, −2)* | −20 (−37, −2)* |
| Triglyceride (mg/dL) | 77 ± 41 | 122 ± 102 | −45 (−87, −2)* | −71 (−115, −28)* |
| Bone Density, Vitamin D, Calcium | ||||
| BMD subtotal (g/cm2) | 0.88 ± 0.05 | 1.03 ± 0.11 | −0.15 (−0.19, −0.11)* | −0.17 (−0.21, −0.13)* |
| BMD total (g/cm2) | 1.01 ± 0.06 | 1.16 ± 0.11 | −0.15 (−0.20, −0.11)* | −0.17 (−0.22, −0.13)* |
| 25-hydroxyvitamin D (ng/mL) | 23 ± 8 | 27 ± 9 | −4 (−8, −0.5)* | −4 (−7, 0) |
| Calcium (mg/dL) | 9.3 ± 0.3 | 9.6 ± 0.3 | −0.2 (−0.4, −0.1)* | −0.3 (−0.4, −0.1)* |
2-hour glucose values were available in only 31 (35%) NHANES participants. 3 patients with WS and known diabetes did not undergo OGTT, and an additional patient with WS did not have adequate venous access for OGTT.
Indicates p < 0.05 for comparison between WS and controls
Figure 1:
Relationship between body mass index and total body fat mass (A) and lean mass (B) in WS (O) and Control (+) subjects. The association between increasing BMI and increasing fat mass is not different between WS and controls, but there is a significant difference in the association between increasing BMI and increasing lean mass (p-value = 0.008 for interaction between BMI and Williams syndrome status).
In analysis by sex, there remained marked reductions in lean mass in both males and females with WS compared to controls; this difference appeared especially pronounced for males (Supplemental Table 1). Percent fat was higher in both males and females with WS, and tended to remain higher following adjustment for BMI, but adjusted subgroup analyses did not reach statistical significance.
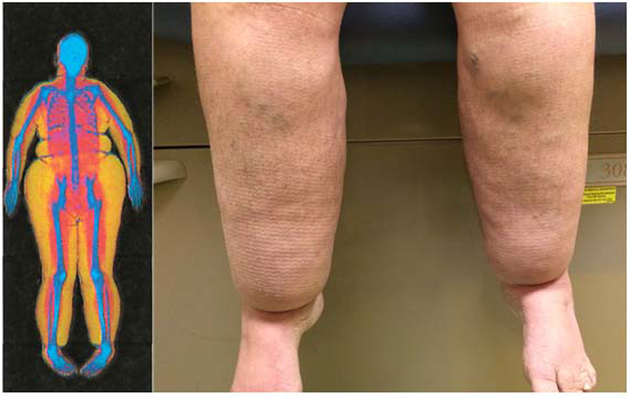
After adjustment for BMI, body fat distribution, as assessed by android:gynoid ratio and trunk:limb fat ratio, were similar among WS subjects and controls. Four of the WS subjects, two males and two females, were clinically judged to have disproportionate lower extremity fat accumulation on exam with a lipedema-like phenotype. Anthropometric, metabolic, and hormonal parameters for these patients are shown in Table 2. Of these patients, two had severe lower-extremity fat accumulation with cuffing at the ankles. DXA scan and photo of one of these patients is shown in Figure 2.
Table 2:
Anthropometric and Metabolic Parameters for 4 WS subjects with Moderate to Severe Lipedema-like Phenotype
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Severity of lower extremity fat deposition | Moderate | Moderate | Severe | Severe |
| Sex | F | M | F | M |
| Age group (y)a | 20–25 | 35–40 | 40–45 | 45–50 |
| Body Mass Index (kg/m2) | 33 | 39 | 34 | 52 |
| Waist circumference (cm) | 88.8cm | 124.6cm | 93.4cm | 138cm |
| Waist:Hip Ratio | 0.79 | 0.98 | 0.76 | 0.93 |
| Percent body fat (%) | 44 | 45 | 53 | 50 |
| Trunk-to-limb fat ratio | 0.80 | 1.12 | 0.79 | 0.98 |
| Resting Energy Expenditure (kcal/day) | 1291 | 1303 | 1243 | 1643 |
| % Predicted Resting Energy Expenditure | 86 | 64 | 99 | 78 |
| Glycemic status | IFG and IGT | Normal | Known DM | Known DM |
| Total cholesterol (mg/dL) | 199 | 160 | b | 163 |
| HDL (mg/dL) | 63 | 59 | b | 51 |
| LDL (mg/dL) | 125 | 84 | b | 85 |
| Triglyceride (mg/dL) | 55 | 87 | b | 135 |
| Estradiol (pg/mL) | 156 | 37.2 | b | 136 |
| Estrone (pg/mL) | 114 | 61.7 | b | 65.6 |
| Total testosterone (ng/dL) | 71 | 291 | b | 210 |
| Sex hormone binding globulin (nmol/L) | 42 | 24 | b | 63 |
| TSH (μU/mL) | 2.01 | 2.34 | b | 2.27 |
| Total Adiponectin (μg/mL) | 9.4 | 4.4 | b | 5.9 |
Ages are provided in a range to protect confidentiality.
This individual did not have adequate venous access for phlebotomy.
Abbreviations: HDL, High Density Lipoprotein-Cholesterol; LDL, Low Density Lipoprotein-Cholesterol; TSH, thyroid stimulating hormone; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; HbA1c, hemoglobin A1c.
Assay-specific normal ranges for hormonal parameters are as follows: estradiol, 10–50 pg/mL in males, 30–400 pg/mL in premenopausal females; estrone, 10–60 pg/mL in males, 17–200 pg/mL in premenopausal females; total testosterone, 270–1070 ng/mL in males, 15–70 in females; sex hormone binding globulin, 13.3–89.5 nmol/L in males, 18.2–135.5 nmol/L in females; TSH 0.5–4 μU/mL. Normal ranges are not available for adiponectin.
Figure 2:
Whole body DXA scan (left) and photo (R) of a female patient with lipedema-like phenotype (Patient #3 in Table 2). In the DXA scan, yellow coloring denotes fat mass, red denotes muscle, and blue denotes bone.
Glucose and Lipid Metabolism
Three subjects with WS had known diabetes, two of whom were taking metformin. No other individuals with WS were receiving any glucose lowering medications. Of the 19 remaining subjects with WS, none had diabetes on testing, but 12 (63%) had some evidence of abnormal glucose homeostasis (Supplemental Table 2). In comparison to NHANES controls, HbA1c (+0.5% [95% CI 0.2, 0.7]) and 2-hour glucose (+68mg/dL [95% CI 44, 93]) were significantly higher in WS, whereas fasting blood glucose was not significantly different (Table 1). Modeling of 2-hour glucose by body composition showed that lean mass was inversely associated with 2-hour glucose levels (−1.3mg/dL for each 1kg increase in lean mass) whereas fat mass was positively associated (+1.4mg/dL for each 1kg increase in fat mass). The presence of WS was not an effect modifier of these relationships.
Total cholesterol, triglycerides (TGL), and low density lipoprotein (LDL) were all significantly lower in WS vs. controls (Table 1), whereas there was no difference in HDL-cholesterol. Four individuals with WS were taking statins. Excluding these individuals and their matched controls from the analysis, the difference in triglyceride between groups persisted, whereas the differences in total cholesterol and LDL were no longer statistically significant. Of note, even after excluding statin users with WS and their matched controls, the expected relationship between increased body fat and increased LDL and triglyceride that is seen in the general population was not seen in WS (Figures 3A and 3B). The presence of WS significantly modified the effect of body fat on both LDL (p = 0.03 for interaction of WS x body fat, Figure 3A) and triglyceride (p = 0.04 for interaction term, Figure 3B).
Figure 3:
Relationship between total body fat and LDL-cholesterol (A) and fasting triglyceride (B) in WS (O) and Control (+) subjects. There is a significant interaction between total body fat and Williams syndrome status (for interaction term, p = 0.03 for LDL, p = 0.04 for triglyceride), such that the expected increase in LDL and triglyceride with increasing body fat is not seen in Williams syndrome.
Bone Density, Calcium, and Vitamin D
Total body BMD and total-body-less-head (subtotal) BMD were significantly lower in individuals with WS compared to controls (Table 1). This difference persisted after adjusting for BMI, as shown in Table 1, and was present in both males and females (Supplemental Table 1). When adjusting for height (without adjustment for BMI) the difference in BMD between groups was attenuated but remained significant (effect sizes −0.043 [−0.084, −0.001] for total-body-less head and −0.063 [−0.110, −0.016] for total body BMD). As shown in Table 1, serum Vitamin D concentrations were lower in WS compared to controls, and calcium was lower, although not to a clinically meaningful degree. Eighty-one percent of WS patients had serum 25-hydroxyvitamin D levels less than 30ng/mL, compared to 66% percent of NHANES controls; the percentage of individuals with 25-hydroxyvitamin D <20ng/mL was 28% in WS and 23% in controls.
Bone markers and sex steroid concentrations were measured in the individuals with WS (Table 3). Reference values for NHANES controls were not available. In the WS cohort, estrone (r = 0.52, p = 0.02) was significantly positively associated with total BMD, with a similar association for subtotal BMD. In subgroup analyses by sex, the correlation coefficient for estrone and total BMD was similar in women (r = 0.63) and men (r = 0.62), but the association in men only trended toward significance (p = 0.07) whereas it remained significant in subgroup analysis for women (p = 0.03). Parathyroid hormone and bone turnover markers were not significantly associated with total or subtotal BMD (data not shown).
Table 3:
Bone Markers and Hormonal Parameters in Men and Women with Williams Syndrome
| Males (N=9) | Females (N=12)a | |||||
|---|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Min, Max | Mean ± SD | Median (IQR) | Min, Max | |
| PTH (pg/mL) | 31 ± 18 | 25 (18, 41) | 13, 64 | 43 ± 17 | 46 (26, 53) | 19, 74 |
| P1NP (μg/L) | 60 ± 26 | 57 (49, 59) | 19, 107 | 64 ± 22 | 54 (50, 76) | 42, 118 |
| BSAP (μg/L) | 9.8 ± 2.7 | 9.8 (8.1, 11.8) | 5.4, 13.1 | 9.4 ± 1.5 | 9.6 (8.5, 10.5) | 6.1, 11.4 |
| CTX (ng/mL) | 0.51 ± 0.27 | 0.50 (0.32, 0.77) | 0.14, 0.9 | 0.54 ± 0.20 | 0.51 (0.38, 0.69) | 0.30, 0.89 |
| Plasma NTX (nM BCE) | 11.3 ± 3.2 | 11.6 (10.4, 12.8) | 5.6, 15.6 | 12.5 ± 2.8 | 12.3 (9.8, 15.0) | 8.4, 16.2 |
| Estrone (pg/mL) | 56 ± 9 | 59 (48, 64) | 40, 66 | 85 ± 62 | 75 (36, 110) | 24, 223 |
| Estradiol (pg/mL) | 45 ± 35 | 33 (29, 45) | 24, 136 | 119 ± 114 | 82 (34, 187) | 6, 386 |
| Total Testosterone (ng/dL) | 483 ± 205 | 409 (307, 646) | 210, 766 | 70 ± 20 | 67 (54, 84) | 45, 107 |
| SHBG (nmol/L) | 50 ±19 | 50 (35, 64) | 24, 75 | 80 ± 43 | 70 (46, 108) | 32, 157 |
| DHEA-S (μg/dL) | 266 ± 59 | 267 (238, 291) | 159, 346 | 169 ± 64 | 158 (120, 195) | 99, 308 |
| Adiponectin (μg/mL) | 6.14 ± 2.07 | 5.88 (4.72, 8.07) | 2.87, 9.10 | 7.72 ± 1.63 | 7.92 (6.12, 9.24) | 5.44, 9.79 |
Blood sampling could not be performed for one of the women in the study.
Abbreviations: SD: standard deviation; IQR, interquartile range; Min, minimum; Max, maximum; PTH, parathyroid hormone; PINP, intact aminoterminal propeptide of type I procollagen; BSAP, bone specific alkaline phosphatase; CTX, serum collagen type 1 cross-linked C- telopeptide; NTX, serum collagen type 1 cross-linked N- telopeptide; SHBG, sex hormone binding globulin; DHEA-S, dehydroepiandrosterone-sulfate.
Parathyroid hormone (PTH), bone specific alkaline phosphatase (BSAP), and plasma n-terminal telopeptide (NTX) were within the assay-specific normal ranges for all WS participants. Six individuals (3M, 3F) had elevated CTX, and 5 participants (2M, 3F) had elevated PINP, but these abnormalities did not correlate with lower total body BMD scores.
Hormonal Parameters
Thyroid stimulating hormone (TSH) and free thyroxine (free T4) were normal for all individuals with WS, but 4 of the 22 individuals studied were receiving levothyroxine for hypothyroidism.
With regard to gonadal steroids, one male with severe lipedema-like phenotype was frankly hypogonadal and also had elevated estrone and estradiol (see Table 2, patient 4). Another male had a borderline testosterone level (between 270–300 ng/mL) and elevated estrone, but normal estradiol (see Table 2, patient 2). Testosterone was normal in the other 7 men studied, but estrone was above the assay reference range for 2 additional men with WS, for a total of 4 of 9 men studied presenting with elevated estrone levels.
Five of the females studied were receiving some form of gonadal steroid treatment (either orally or through an intrauterine device). Of the remaining seven, sex hormone binding globulin, estradiol, and estrone levels were normal, but five had modest elevations in total testosterone (>70ng/mL).
Resting Energy Expenditure
Indirect calorimetry in individuals with WS showed that all individuals had a resting energy expenditure (REE) below that predicted by sex, weight, height, and age, with mean percent predicted REE of 84.6±9.5%.
Discussion
Our data indicate lower lean mass in WS individuals in spite of higher BMI, as well as an increased body fat percentage in WS compared to controls. Hemoglobin A1c and 2-hour blood glucose levels from an OGTT were elevated in patients with WS. After accounting for body composition differences, individuals with WS had lower triglyceride than controls. Finally, we show reduced BMD in both male and female individuals with WS, a difference that persists when adjusting for shorter stature in this group. Our sample size was not large enough to draw conclusions about associations between bone markers, hormone levels, and the metabolic or bone phenotype of WS; however, our data show abnormal bone formation and bone turnover markers in some patients and also suggest that men with WS may benefit from testing for hypogonadism whereas women with WS may have relatively high levels of total testosterone.
To our knowledge, reductions in lean mass have not previously been described in adults with WS, and these results have important clinical implications. Reduced lean mass may explain the relative decrease in resting energy expenditure in this population, which may, in turn, predispose to the excessive weight gain in adulthood that we have previously described in WS (2). Lower lean mass is also associated with increased 2-hour glucose and may explain part of the predisposition to IGT in individuals with WS. Finally, lower lean mass may have important implications for physical function and for frailty in older age, possibilities that will need to be explored in future research.
The high percentage of impaired glucose metabolism (characterized by impaired fasting glucose, IGT, and/or elevated HbA1c) in our cohort is in agreement with previous studies (1, 8–11). In the present cohort, 63% of those without diabetes had some evidence of impaired glucose metabolism, and this cohort was relatively younger (mean age of 28.5 vs. 35y) than our previously described group with an IGT or DM frequency of 75%. Lunati et al. recently described the evolution of glycemia over 5 years in 31 young adults with WS of similar age to our current cohort. In that cohort, of 30 adults without known diabetes at baseline, initial testing showed DM in 3 patients and impaired glucose metabolism in 15 patients (8). After 5 years, repeat testing showed DM in 5 patients and impaired glucose in 14 patients, suggesting progression of dysglycemia over time in this population (8). Haploinsufficiency of two genes in the deleted region in WS, syntaxin 1A (STX-1A) and carbohydrate response element-binding protein (ChREBP), may be largely responsible for the phenotype of dysglycemia in WS. STX-1A, involved in vesicle docking and function, is a regulator of insulin secretion (4, 12), whereas ChREBP is upregulated in the presence of glucose, increases the transcription of multiple genes involved in glucose metabolism, and is thus a major regulator of whole body insulin sensitivity (3, 13). Of note, the diabetes in WS most closely resembles a Type 2 diabetes phenotype, with negative islet autoantibodies and laboratory assessment consistent with insulin resistance (1, 10, 14). Traditional risk factors for Type 2 diabetes are not always present in individuals with WS and diabetes, however, and, given that the underlying genetic defect may alter both insulin secretion and insulin sensitivity, it may not be appropriate to classify the diabetes in WS as either Type 1 or Type 2. Clinically, our results support the need for regular surveillance of glucose metabolism in adults with WS.
Our finding of reduced LDL and triglyceride in WS compared to controls reproduces and extends the findings of Palacios-Verdú et al., who described relatively low triglycerides in WS (15). We demonstrate that the expected relationship between increasing body fat and increased LDL and triglyceride is not present in WS, suggesting a role for the underlying genetics of WS. ChREBP upregulates the transcription of many enzymes involved in triglyceride synthesis (16), and haploinsufficiency of ChREBP may be responsible for the lipid phenotype in WS.
All the individuals in our cohort had a resting energy expenditure (REE) <100% of that predicted by the Harris-Benedict equation, with the average REE in WS being only 86.5% of that predicted. Though we suspect this is, at least in part, due to the reduced lean mass in WS, the absence of indirect calorimetry data in controls leaves us unable to assess that hypothesis. Our findings contrast with those of Kaplan et al., who reported a higher REE and percent predicted REE, as well as lower body fat, in three adults with WS compared to three gender-matched adult controls (17). Given the small sample size of the Kaplan et al. cohort and the lack of matched control data in our cohort, REE should be further studied in adults with WS.
Our findings that whole body BMD is lower in both males and females with WS compared to controls are consistent with previous data from several studies (2, 9, 18). To better understand factors that may contribute to this discrepancy, we examined, calcium, vitamin D, PTH, as well as bone markers in WS. Mean serum calcium was modestly lower and vitamin D tended to be lower in WS compared to NHANES controls, but the clinical significance of these small differences is likely minimal. None of the serum calcium levels in members of our adult WS cohort were out of the normal range. Historically, hypercalcemia was viewed as a prominent feature of WS, particularly in childhood. Recent data collected by Sindhar et al. (19) provides new insights in calcium issues. Specifically, Sindhar et al. demonstrated normal serum calcium levels in the clear majority of adults with WS over 20 years of age. While a small number had hypercalcemia, it was generally due to a secondary cause besides WS. Sindhar et al. additionally noted hypocalcemia in several adults (19), confirming the findings of an earlier adult study (9). Accordingly, the minor decrease in our mean WS calcium level compared to the NHANES control mean, with all WS values remaining in the range of normal, is consistent with published adult data and, further, we believe, is not clinically meaningful.
Sindhar et al. did observe “actionable” hypercalcemia in 5% and 10% of infants and toddlers, respectively. In light of the historical view that infantile hypercalcemia was highly prevalent, this may have prompted individuals with WS who are now adults to follow diets limited in calcium and vitamin D to prevent or treat hypercalcemia. In turn, these dietary restrictions could contribute to the lower BMD we observe in this population. Unfortunately, we do not have data in our adult cohort regarding whether such diets were followed in childhood. We concur with the recommendation of Sindhar et al. (19) that calcium and vitamin D intake should not be restricted in individuals with WS and normal calcium levels
With regard to vitamin D levels, it is important to note that the higher percent fat mass in WS may be responsible for lower vitamin D, as vitamin D is stored in adipose tissue, and serum levels are inversely related to BMI in the general population (5).
PTH was normal in all the WS individuals studied. CTX and P1NP were abnormal in a few subjects, and study of larger cohorts is needed to investigate if these abnormalities are clinically relevant and if they may be related to underlying genetics. Genes in the WS critical region that potentially contribute to low bone mass include LIMK1 and FZD9. LIMK1 is a serine/threonine kinase that plays a role in cytoskeletal remodeling (5). It is expressed in both osteoblasts and osteoclasts, and two studies have suggested an important role in osteoblast differentiation (5, 14). LIMK1 null mice demonstrate a 5% reduction in total body BMD compared to controls (5). Osteoblasts in LIMK1 null mice show reduced number and function, whereas osteoclasts are normal in number and demonstrate increased resorptive activity compared to LIMK+/− osteoclasts (5). Serum CTX also tended to be higher in the knock-out animals compared to controls (5). FZD9 is a Wnt receptor that promotes bone remodeling through non-canonical Wnt signaling (19, 20). FZD9 knock-out mice demonstrate reduced BMD, with reduced osteoblast number and impaired bone formation but no apparent abnormalities in osteoclast function (20). Haploinsufficiency of FZD9 in mice, equivalent to its hemizygous deletion in WS, also leads to decreased BMD, lower number of osteoblasts, and reduced bone formation (20).
Lifestyle factors including levels of weight-bearing physical activity play an important role in determining BMD (21, 22), but we were unable to collect reliable physical activity data in our cohort. In our clinical observations, individuals with WS are probably, on average, less physically active than the general population, and this certainly may contribute to reduce BMD. It remains unknown whether individuals with WS have a higher fracture risk than the general population.
The presence of clinically evident lipedema in 4 members of our WS cohort (18%) is similar to the frequency observed in WS adults from two previously published cohorts (2, 9). We likewise confirmed the presence of lipedema in males with WS, in contrast to its near total absence among males in the general population. Due to the relatively small number of individuals with lipedema in our cohort, we cannot determine if the presence of lipedema is associated with dysglycemia, altered lipid profile, or other metabolic or hormonal abnormalities. The two males with lipedema did have low testosterone levels and relatively high estrone levels, however, suggesting a potential contribution of altered gonadal steroids.
One of our study’s major strengths is the breadth of metabolic and clinical phenotyping data collected on 22 individuals with WS, an uncommon genetic disorder. We believe the data presented herein demonstrate novel observations (regarding fat and muscle composition in adults with WS that have potential health impact), and further contribute to the evolving understanding of the hormonal, metabolic, and body composition phenotype in WS. Our study, however, also has several limitations. Our results may be limited by sample size and the relatively young age of participants in our cohort and, therefore, not be applicable to older adults with WS. All data on subjects with WS were collected at MGH, whereas control data were extracted from the NHANES dataset, with more limited availability and some differences in laboratory methodologies. Accordingly, comparison between WS and controls may have inherent bias that is difficult to characterize or quantify. Additionally, due to lack of hormonal parameters in NHANES we were unable to assess whether gonadal hormones and bone turnover markers differed in WS compared to controls. We have data on whole body and subtotal (whole body less head) BMD, but do not have data for site-specific BMD measurements such as the hip, forearm, or lumbar spine. While both WS and NHANES DXA scans were performed on Hologic bone densitometers, they were done on different models (Discovery A vs. QDR-4500A, respectively), potentially leading to minor systematic differences between results. Although the magnitude of differences we show between WS and controls are unlikely to be entirely attributable to differences between models, it will be important to validate these findings using WS and control individuals scanned on the same machine, and potentially using newer techniques such as high-resolution peripheral quantitative CT, which are not affected by differences in height or body mass. Finally, absence of detailed data regarding medication use for NHANES controls prevented adjustment of BMD analyses for use of hormonal supplements as well as full adjustment for anti-diabetic or lipid-lowering medications for the metabolic parameters.
Overall, our results demonstrate for the first time a reduction in lean body mass in adults with WS, and we add to previous reports of impaired glucose metabolism, reduced triglyceride and LDL, and reduced bone mineral density. These results should inform clinical recommendations for adults with WS to engage in regular physical activity to preserve lean mass, improve glucose tolerance, and maintain BMD, particularly as they age. Our results also highlight the importance of being aware of vitamin D status and bone health in this population. Further studies are needed in multiple areas for individuals with WS, including investigation of whether fracture risk is increased, whether dysglycemia confers cardiovascular risk or whether individuals are protected by low LDL, and how the phenotypic findings in WS may be used to elucidate the clinical importance of genes in the deleted region.
Supplementary Material
Acknowledgements:
We express sincere appreciation for the individuals and their families who participated in this research as well as the staff of the MGH Clinical Research Center. Funding was provided, in part, by NIH P30 DK057521 (S.S.), P30 DK040561 (T.S.), 1UL1TR001102, and Department of Medical Sciences, Frank H Netter School of Medicine (BRP).
Footnotes
Conflict of Interest: None of the authors have a conflict of interest to declare.
References
- 1.Pober BR, Wang E, Caprio S, Petersen KF, Brandt C, Stanley T, et al. High prevalence of diabetes and pre-diabetes in adults with Williams syndrome. Am J Med Genet C Semin Med Genet. 2010. May 15;154C(2):291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waxler JL, Guardino C, Feinn RS, Lee H, Pober BR, Stanley TL. Altered body composition, lipedema, and decreased bone density in individuals with Williams syndrome: A preliminary report. Eur J Med Genet. 2017. May;60(5):250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci U S A. 2004. May 11;101(19):7281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam PP, Leung YM, Sheu L, Ellis J, Tsushima RG, Osborne LR, et al. Transgenic mouse overexpressing syntaxin-1A as a diabetes model. Diabetes. 2005. September;54(9):2744–54. [DOI] [PubMed] [Google Scholar]
- 5.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000. September;72(3):690–3. [DOI] [PubMed] [Google Scholar]
- 6.Harris JA, Benedict FG. A Biometric Study of Human Basal Metabolism. Proc Natl Acad Sci U S A. 1918. December;4(12):370–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grandits G, Neuhaus J. Paper 061–2010: Using SAS to Perform Individual Matching in Design of Case-Control Studies. SAS Global Forum 2010; Seattle, WA: 2010. [Google Scholar]
- 8.Lunati ME, Bedeschi MF, Resi V, Grancini V, Palmieri E, Salera S, et al. Impaired glucose metabolism in subjects with the Williams-Beuren syndrome: A five-year follow-up cohort study. PLoS One. 2017;12(10):e0185371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherniske EM, Carpenter TO, Klaiman C, Young E, Bregman J, Insogna K, et al. Multisystem study of 20 older adults with Williams syndrome. Am J Med Genet A. 2004. December 15;131(3):255–64. [DOI] [PubMed] [Google Scholar]
- 10.Stagi S, Lapi E, Cecchi C, Chiarelli F, D’Avanzo MG, Seminara S, et al. Williams-beuren syndrome is a genetic disorder associated with impaired glucose tolerance and diabetes in childhood and adolescence: new insights from a longitudinal study. Horm Res Paediatr. 2014;82(1):38–43. [DOI] [PubMed] [Google Scholar]
- 11.Masserini B, Bedeschi MF, Bianchi V, Lunati ME, Lalatta F, Beck-Peccoz P, et al. High prevalence of impaired glucose metabolism in young adult patients with Williams syndrome European Congress of Endocrinology 2011; 30 April 2011 – 04 May 2011; Rotterdam, The Netherlands: 2011. [Google Scholar]
- 12.Pasyk EA, Kang Y, Huang X, Cui N, Sheu L, Gaisano HY. Syntaxin-1A binds the nucleotide-binding folds of sulphonylurea receptor 1 to regulate the KATP channel. J Biol Chem. 2004. February 6;279(6):4234–40. [DOI] [PubMed] [Google Scholar]
- 13.Herman MA, Peroni OD, Villoria J, Schon MR, Abumrad NA, Bluher M, et al. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature. 2012. April 19;484(7394):333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masserini B, Bedeschi MF, Bianchi V, Scuvera G, Beck-Peccoz P, Lalatta F, et al. Prevalence of diabetes and pre-diabetes in a cohort of Italian young adults with Williams syndrome. Am J Med Genet A. 2013. April;161A(4):817–21. [DOI] [PubMed] [Google Scholar]
- 15.Palacios-Verdu MG, Segura-Puimedon M, Borralleras C, Flores R, Del Campo M, Campuzano V, et al. Metabolic abnormalities in Williams-Beuren syndrome. J Med Genet. 2015. April;52(4):248–55. [DOI] [PubMed] [Google Scholar]
- 16.Yamashita H, Takenoshita M, Sakurai M, Bruick RK, Henzel WJ, Shillinglaw W, et al. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc Natl Acad Sci U S A. 2001. July 31;98(16):9116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan AS, Stallings VA, Zemel BS, Green KA, Kaplan P. Body composition, energy expenditure, and energy intake in patients with Williams syndrome. J Pediatr. 1998. February;132(2):223–7. [DOI] [PubMed] [Google Scholar]
- 18.Stagi S, Manoni C, Scalini P, Chiarelli F, Verrotti A, Cecchi C, et al. Bone mineral status and metabolism in patients with Williams-Beuren syndrome. Hormones (Athens). 2016. July 11. [DOI] [PubMed] [Google Scholar]
- 19.Sindhar S, Lugo M, Levin MD, Danback JR, Brink BD, Yu E, et al. Hypercalcemia in Patients with Williams-Beuren Syndrome. J Pediatr. 2016. November;178:254–60 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albers J, Schulze J, Beil FT, Gebauer M, Baranowsky A, Keller J, et al. Control of bone formation by the serpentine receptor Frizzled-9. J Cell Biol. 2011. March 21;192(6):1057–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alghadir AH, Gabr SA, Al-Eisa E. Physical activity and lifestyle effects on bone mineral density among young adults: sociodemographic and biochemical analysis. J Phys Ther Sci. 2015. July;27(7):2261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braun SI, Kim Y, Jetton AE, Kang M, Morgan DW. Prediction of bone mineral density and content from measures of physical activity and sedentary behavior in younger and older females. Prev Med Rep. 2015;2:300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.