Significance
Serine supports a number of anabolic processes, including protein, lipid, and nucleic acid synthesis. Cells can either import serine or synthesize it de novo. Recently, overexpression of 3-phosphoglycerate dehydrogenase (PHGDH), the gene encoding the first committed step of serine synthesis, via focal amplification and other mechanisms, has been identified in human cancers. Cancer cell lines that overexpress PHGDH are uniquely sensitive to PHGDH knockdown whereas lines that express little PHGDH are insensitive, suggesting that PHGDH may be a clinically interesting target. Here, we report the discovery of a specific small molecule inhibitor of PHGDH, which enables preclinical evaluation of PHGDH as a target in cancer and provides a tool to study the biology of de novo serine synthesis.
Keywords: PHGDH, inhibitor, serine, cancer metabolism
Abstract
Cancer cells reprogram their metabolism to promote growth and proliferation. The genetic evidence pointing to the importance of the amino acid serine in tumorigenesis is striking. The gene encoding the enzyme 3-phosphoglycerate dehydrogenase (PHGDH), which catalyzes the first committed step of serine biosynthesis, is overexpressed in tumors and cancer cell lines via focal amplification and nuclear factor erythroid-2-related factor 2 (NRF2)-mediated up-regulation. PHGDH-overexpressing cells are exquisitely sensitive to genetic ablation of the pathway. Here, we report the discovery of a selective small molecule inhibitor of PHGDH, CBR-5884, identified by screening a library of 800,000 drug-like compounds. CBR-5884 inhibited de novo serine synthesis in cancer cells and was selectively toxic to cancer cell lines with high serine biosynthetic activity. Biochemical characterization of the inhibitor revealed that it was a noncompetitive inhibitor that showed a time-dependent onset of inhibition and disrupted the oligomerization state of PHGDH. The identification of a small molecule inhibitor of PHGDH not only enables thorough preclinical evaluation of PHGDH as a target in cancers, but also provides a tool with which to study serine metabolism.
Serine is required for a plethora of anabolic processes. Serine is an abundant component of proteins and is required for the synthesis of lipids, including sphingolipids and phosphatidylserine, a major component of cellular membranes (1–3). Alternatively, serine hydroxymethyltransferases (SHMTs) convert serine to glycine, concomitantly charging the folate pool with “one-carbon” units (4, 5). Both glycine and folate one-carbon units are used to make nucleotides. Thus, serine serves numerous critically important roles in cellular metabolism.
At the cellular level, serine can be imported from the extracellular space via amino acid transporters (6, 7). Alternatively, serine can be synthesized from glucose via the phosphoserine pathway (8). De novo synthesis proceeds from the glycolytic intermediate 3-phosphoglycerate (3-PG) via three sequential enzymatic reactions (Fig. 1A), the first of which is catalyzed by the NAD+-dependent enzyme 3-phosphoglycerate dehydrogenase (PHGDH) (9). For decades, it has been known that cancer cells have enhanced serine synthesis, which contributes to nucleotide synthesis (10, 11). Recently, focal amplifications of the gene encoding PHGDH have been reported, particularly in breast cancers and melanomas (12–14). Additionally, KEAP1 and nuclear factor erythroid-2-related factor 2 (NRF2) mutant non-small cell lung cancers (NSCLCs) overexpress PHGDH (15). Proliferation of PHGDH-amplified cancer cell lines, and other lines that overexpress PHGDH without amplification, is inhibited by PHGDH knockdown. In contrast, lines that express little PHGDH are resistant to shRNA-mediated ablation of the pathway, presumably because serine import suffices (13, 14). A detailed mechanistic understanding of why some cancer cells are addicted to serine synthesis despite the availability of extracellular serine for import remains unclear. Interestingly, in triple negative breast cancer (TNBC) and NSCLC, PHGDH amplification and overexpression are associated with more aggressive disease (13–16). Thus, PHGDH inhibitors as a targeted therapy for these tumor types represent an exciting clinical opportunity.
Fig. 1.
Screening for inhibitors of PHGDH. (A) Serine synthesis from glucose via the phosphoserine pathway: Phosphoglycerate dehydrogenase (PHGDH) oxidizes the glycolytic intermediate 3-phosphoglycerate (3-PG) to 3-phosphohydroxypyruvate (p-Pyr) using NAD+; phosphoserine amino transferase (PSAT1) transaminates p-Pyr to phosphoserine (p-Ser) using glutamate as a nitrogen donor; phosphoserine phosphatase (PSPH) dephosphorylates p-Ser to yield serine. (B) In vitro PHGDH assay. Diaphorase couples the NADH produced upon PHGDH turnover to the reduction of resazurin to fluorescent resorufin. Resorufin fluorescence is a proxy for PHGDH activity. PSAT1 is included to prevent product feedback inhibition of PHGDH by p-Pyr. (C) Z-score plot for the 800,000-compound library screened using the above PHGDH assay. Each point represents a single compound. A negative score indicates inhibition. (D) Screen triaging strategy. Setting a Z-score threshold of −3 gave 3,906 putative hits. After counter-screening against diaphorase to rule out false positives and confirming activity against PHGDH, 408 compounds remained. Selected compounds were profiled against a panel of metabolic NAD(P)+ dehydrogenases to ascertain selectivity for PHGDH. (E) Structures of representative PHGDH inhibitors evaluated in cell-based assays.
The studies herein detail our efforts in identifying small molecule inhibitors of PHGDH. We reasoned that a PHGDH inhibitor would have the benefits of not only providing a tool compound with which to study the biology of serine synthesis, but also enabling thorough preclinical evaluation of PHGDH as a target in cancers. We screened a library of 800,000 small molecules using an in vitro PHGDH assay. A cell-based assay for serine synthesis was used to identify a lead, CBR-5884, that was active in cells. CBR-5884 selectively inhibited the proliferation of melanoma and breast cancer lines that have a high propensity for serine synthesis but had no effect on lines that rely on extracellular serine uptake. Mechanistically, CBR-5884 was found to be a noncompetitive inhibitor, showed a time-dependent onset of inhibition, and disrupted the oligomerization state of PHGDH.
Results
Screening for Small Molecule Inhibitors of PHGDH.
An in vitro enzymatic assay for PHGDH activity amenable to high-throughput screening (HTS) was developed by coupling the production of NADH, upon 3-PG oxidation, to the reduction of resazurin to resorufin using diaphorase as the coupling enzyme (Fig. 1B). Thus, resorufin fluorescence served as a proxy for PHGDH activity. The assay was miniaturized to a 1,536-well format with a Z-factor of >0.75, indicating a high quality assay (17). A library of 800,000 small molecules was screened in single point format at 13 μM (Fig. 1C). Setting a threshold Z score of −3, corresponding to at least 50% PHGDH inhibition, gave a 0.5% hit rate, yielding 3,906 hits. Putative hits were reassayed in triplicate and counter-screened against diaphorase to rule out false positives targeting diaphorase. The counter screen eliminated 3,498 compounds, giving 408 PHGDH inhibitors (Fig. 1D).
A triaging strategy based on hit potency and selectivity was designed. We reasoned that inhibitors specific to PHGDH would minimize general cellular toxicity compared with compounds that hit a variety of dehydrogenases. Thus, half maximal inhibitory concentrations (IC50) were determined for a panel of NAD(P)+-dependent dehydrogenases that included PHGDH, isocitrate dehydrogenase (IDH1), malate dehydrogenase (MDH1), and 3α-hydroxysteroid dehydrogenase (3α-HSD). Compounds at least fourfold more selective for PHGDH were progressed for further analysis. Based on this triaging, seven of the most potent PHGDH inhibitors were selected as lead compounds for evaluation in cell-based assays; selected structures are shown in Fig. 1E (Table S1). A number of these compounds are likely to target sulfhydryl groups and may therefore react with a PHGDH cysteine residue. For example, both CBR-5807 and CBR-6936 contain sulfhydryl-reactive disulfide centers. Interestingly, CBR-5807 (Disulfiram) is an approved drug dosed in humans to treat alcoholism and known to inhibit aldehyde dehydrogenase by reacting with sulfhydryl groups (18).
CBR-5884 Inhibits Serine Synthesis in Cells.
We determined whether any of our seven leads inhibited serine synthesis in cancer cells. To do so, we turned to gas chromatography mass-spectrometry (GCMS) with uniformly carbon-13–labeled glucose (13C6-glucose) tracing. Given the isotopic enrichment of serine, it is possible to decouple newly synthesized serine from extracellular serine or serine that was synthesized before tracer addition. Newly synthesized serine has a mass-shift of 3 (M+3) due to the incorporation of glucose-derived 13C via 3-PG. We first investigated the kinetics of serine labeling. Serine labeling plateaued around 6 h, with ∼65% of the serine pool being 13C-labeled (Fig. S1). The plateau phase likely reflects exchange between intra- and extracellular serine pools (19).
Fig. S1.
Activity of PHGDH CBR-5884 in cells. (A) Kinetics of serine labeling in Carney cells. 13C6-glucose was added at time t = 0, and cells were labeled for the indicated time points. Polar metabolites were extracted and analyzed by GCMS. Glucose-derived serine (M+3 serine) relative to total serine levels is plotted. Each point indicates an independent experiment. (B) In vitro enzymatic assay for malate dehydrogenase 1 (MDH1) or PHGDH titrating CBR-5884 (parent) or the carboxylic acid derivative (acid). Structures of parent and acid are provided for comparison. Initial rates (Vi) normalized to the DMSO control rate (Vo) are plotted. (C and D) Carney cells were treated with a serial dilution of CBR-5884 for 3 h to mimic the acute 13C6-glucose–labeling assay conditions as in Fig. 2A, and cellular reducing potential and ATP levels were determined using an Alamar Blue or ATPGlo assay, respectively. (E) Glycolytic metabolite levels for Carney cells pretreated with DMSO or CBR-5884 (30 μM) for 4 h and then labeled for 2 h with 13C6-glucose under continued DMSO or drug treatment. Polar metabolites were extracted and analyzed by LC-MS/MS. The fractional labeling (M+3 metabolite level relative to total metabolite level) is plotted for each indicated metabolite. Error is given as ±1 SD, n ≥ 3. BPG, 1,3-bisphosphoglycerate or 2,3-bisphosphoglycerate (isotopomers); DHAP, dihydroxy-acetone-phosphate; FBP, fructose-1,6-bisphosphate; F6P, fructose-6-phosphate; G3P, glyceraldehyde-3-phosphate; PEP, phosphoenolpyruvate; 3-PG, 3-phosphoglycerate.
With an understanding of serine-labeling kinetics, we designed a 13C6-glucose tracing assay to acutely interrogate the effects of compounds on serine synthesis (Fig. 2A). Assaying serine synthesis with a 3-h compound treatment was preferred to longer treatments to guard against false positives that decrease serine labeling by an indirect effect, such as generally compromising cellular viability. Among our lead compounds, CBR-5884 was able to decrease de novo serine synthesis by 30%; the remaining compounds had little effect (Fig. 2B). The dose at which CBR-5884 had an effect on serine labeling was consistent with the in vitro biochemical IC50 of 33 ± 12 μM for PHGDH (Fig. 2C). At such concentrations, CBR-5884 had no effect on two other NAD+-dependent dehydrogenases, lactate dehydrogenase (LDH) and MDH1 (Fig. 2C and Fig. S1). Importantly, under the acute treatment time period used in the labeling assays, CBR-5884 was not generally cytotoxic at concentrations up to 40 μM as determined by two independent cellular viability assays (Fig. S1). Therefore, decreases in serine labeling are a direct effect of CBR-5884–mediated PHGDH inhibition.
Fig. 2.
CBR-5884 inhibits serine synthesis in cells. (A) Acute inhibitor treatment assay schematic. Carney cells are pretreated with drug at 30 μM for 1 h before initiating 13C6-glucose labeling for 2 h still in the presence of drug. Polar metabolites are harvested and analyzed by GCMS. (B) The ability of the seven lead PHGDH inhibitors to block serine synthesis was assayed as in A. 13C6-glucose–derived serine (M+3 serine) relative to total serine levels is plotted. (C) In vitro IC50 assays for PHGDH and lactate dehydrogenase (LDH). Initial rates of the enzymatic reaction (Vi) at the indicated CBR-5884 concentration normalized to that of the DMSO control (Vo) are plotted. (D) CBR-5884 dose–response experiment as in A, but monitoring a panel of phosphoserine pathway and glycolytic metabolites. The y axes indicate the fraction of the indicated metabolite derived from glucose (M+3 metabolite level relative to total metabolite level). Asterisks indicate significant differences vs. DMSO treatment (P < 0.05, t test, n ≥ 3). Error is given as ±1 SD.
We resynthesized CBR-5884 in-house and performed a dose–response experiment for CBR-5884 using the same acute treatment method as above. Serine labeling was significantly decreased at 30 μM and trended toward a decrease at 15 μM (Fig. 2D). Importantly, perturbations in labeling were specific to serine in that neither the PHGDH substrate, 3-PG, nor the end products of glycolysis, pyruvate and lactate, were affected (Fig. 2D). We further confirmed that glycolytic metabolites were unperturbed by CBR-5884 treatment using liquid chromatography mass spectrometry (LC-MS/MS) to interrogate a greater panel of metabolites (Fig. S1). Thus, changes in serine labeling are a direct effect of CBR-5884–mediated PHGDH inhibition and not a consequence of changes in PHGDH substrate levels or general perturbations in glycolytic flux (Fig. 2D). The absence of an effect on lactate labeling was consistent with the in vitro data showing that CBR-5884 does not inhibit LDH under the drug concentrations used. In sum, the data argue that CBR-5884 is able to selectively inhibit serine synthesis in cells.
Given that CBR-5884 is an ethyl ester and therefore susceptible to intracellular esterases, we investigated whether the carboxylic acid derivative of the parent molecule was still active against PHGDH; were the acid less active, it would likely decrease the efficiency of targeting PHGDH in situ. Parent and acid derivatives were equally potent against, and selective for, PHGDH in vitro, suggesting that intracellular deesterification is unlikely to affect CBR-5884 activity (Fig. S1).
CBR-5884 Selectively Inhibits the Proliferation of Cancer Cells with a High Propensity for Serine Synthesis.
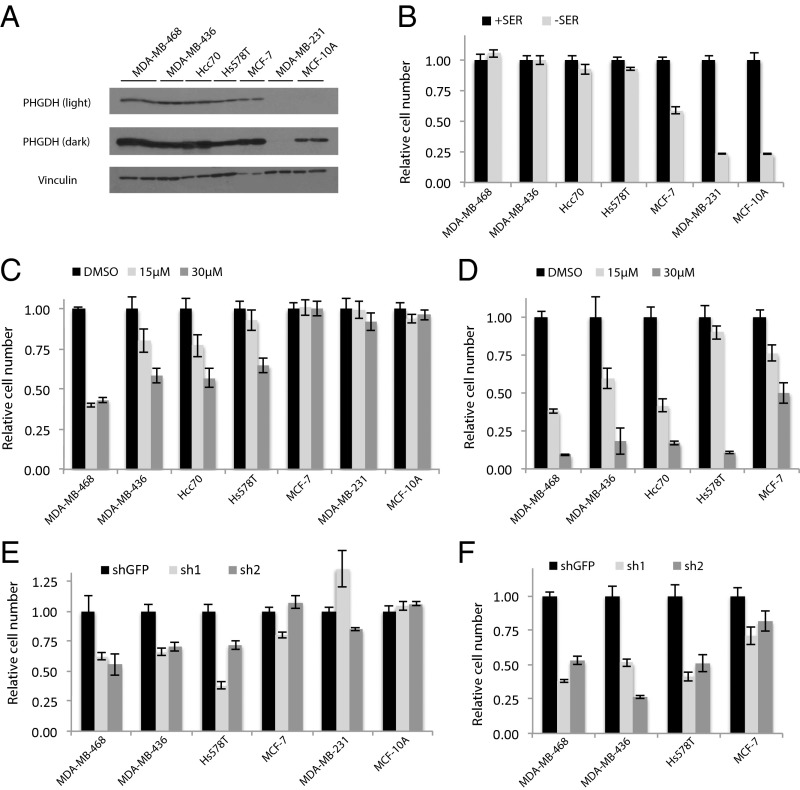
We established a system to test the ability of CBR-5884 to inhibit PHGDH-dependent cancer cell proliferation. We first evaluated the ability of a panel of breast and melanoma cell lines to proliferate in serine replete or deplete media as a proxy for serine biosynthetic activity. Breast lines were selected based on PHGDH expression according to the Cancer Cell Line Encyclopedia (CCLE) data and validated by blotting for PHGDH (Fig. 3A and Fig. S2) (20). Removing extracellular serine had no effect on proliferation of high PHGDH-expressing lines MDA-MB-468, MDA-MB-436, HCC70, and Hs578T (Fig. 3B). All four lines cluster in the top quartile of the CCLE dataset for PHGDH expression (Fig. S2); MDA-MB-468 and HCC70 cells harbor PHGDH amplifications (14). In contrast, serine depletion almost completely abrogated proliferation of low PHGDH-expressing lines MDA-MB-231 and MCF10A (Fig. 3B). In melanoma cells, PHGDH protein levels were similarly commensurate with the ability to proliferate in serine-free media (Fig. S2). Interestingly, although Carney cells are sensitive to extracellular serine depletion, they can adapt and proliferate, as evidenced by increased PHGDH protein levels upon serine depletion (Fig. S2).
Fig. 3.
CBR-5884 selectively inhibits the proliferation of breast cancer lines with a high propensity for serine synthesis. (A) Western blot for lines grown in +SER media: two lanes per cell line with each lane loaded with independent cell lysates. (B) Proliferation assay for breast lines grown in either serine-replete (+SER) or -deplete (−SER) media. Proliferation assay for lines treated with CBR-5884 in (C) +SER or (D) −SER media. Proliferation assay for lines grown in (E) +SER or (F) −SER media with PHGDH knockdown (sh1 and sh2) or a nontargeting control (shGFP). MDA-MB-468 and HCC70 are PHGDH amplified. MCF-10A cells are nontransformed mammary epithelial cells; other lines are cancer cell lines. MDA-MB-231 and MCF-10A lines were not included in −SER experiments in D and F because they are sensitive to serine withdrawal. Histograms depict mean ± SE (n ≥ 3).
Fig. S2.
Relative PHGDH expression and CBR-5884 inhibit growth of melanoma cell lines with a high propensity for serine synthesis. (A) Relative PHGDH expression levels across a panel of 1,036 cell lines obtained from the CCLE dataset (19). Selected breast cancer lines used for drug proliferation assays are highlighted in orange. (B) Western blot for melanoma lines grown in +SER media: two lanes per cell line, with each lane loaded with independent cell lysates. WM266-3 cells are PHGDH-amplified. (C) Proliferation assay for melanoma lines grown in either +SER or −SER media. (D) Western blot for WM266-3 and Carney cells grown in +SER or −SER media: two lanes per cell line, with each lane loaded with independent cell lysates. Proliferation assay for melanoma lines grown in (E) +SER media or (F) −SER media treated with CBR-5884 at 15 μM or 30 μM. Gak cells were not included in the −SER experiment because they cannot grow in −SER media. Proliferation assay for lines grown in (G) +SER media or (H) −SER media with PHGDH knockdown (sh1 and sh2) or a nontargeting control (shGFP). (I) Western blot confirming PHGDH knockdown in breast and melanoma lines used for proliferation assays: PHGDH knockdown (sh1 and sh2) and nontargeting control (shGFP). (J) Proliferation assay for MDA-MB-468 and MCF-10A cells treated with the carboxylic acid derivative of CBR-5884 at 15 μM or 30 μM. Histograms depict mean ± SE (n ≥ 3).
Given that the ability to proliferate in the absence of extracellular serine is indicative of a high propensity for serine synthesis, we hypothesized that such lines should be sensitive to CBR-5884. Conversely, lines that cannot grow in serine-free media have a low propensity for serine synthesis and should therefore be resistant to PHGDH inhibition. Treating the breast lines with CBR-5884 in serine-replete media inhibited growth of the four lines that grew without extracellular serine in a dose-dependent manner, with growth inhibition ranging from 35% to 60% at 30 μM CBR-5884. The inhibitor had no effect on the three lines sensitive to serine withdrawal, indicating that the inhibitor was selectively toxic to cells with high serine synthesis activity (Fig. 3C). We next asked whether removing serine from the media, to enhance the reliance on de novo serine synthesis, could sensitize cells to PHGDH inhibition. Indeed, serine depletion increased the efficacy of CBR-5884 in lines already sensitive under serine-replete conditions as evidenced by an 80–90% decrease in proliferation with 30 μM CBR-5884 (Fig. 3D). Moreover, MCF7 cells, which were of intermediate sensitivity to serine withdrawal, and insensitive to drug under serine-replete conditions, became partially sensitive to the inhibitor under serine-deplete conditions (Fig. 3D). Importantly, under serine-replete conditions, PHGDH knockdown phenocopied the effects of CBR-5884 treatment in that the drug-sensitive lines were also sensitive to PHGDH knockdown (Fig. 3E and Fig. S2). Furthermore, as with the drug treatments, growing cells in serine-free media enhanced the growth inhibitory effect of PHGDH knockdown (Fig. 3F). Similar trends were observed for the melanoma panel in terms of both the selectivity of CBR-5884 for cells with a high propensity for serine synthesis and the increased efficacy under serine-deplete conditions (Fig. S2). Finally, the acid derivative of compound 5884 was not effective on MDA-MB-468 cells, likely owing to poor membrane permeability, and is therefore not a viable alternative to the parent compound (Fig. S2).
Analysis of CBR-5884 Inhibition Modality.
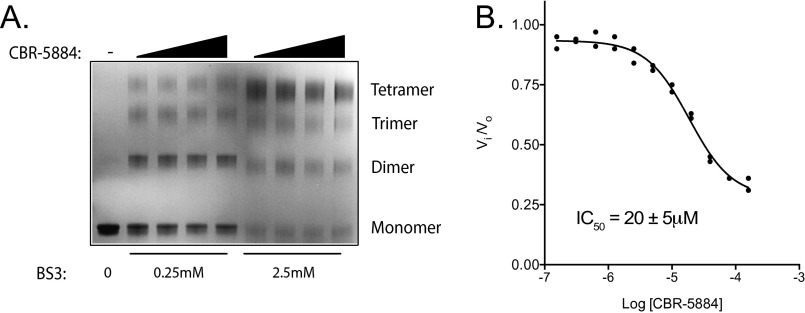
We sought to more deeply characterize the mechanism by which CBR-5884 inhibits PHGDH. Inhibition constants (Ki) for CBR-5884 with respect to each substrate were determined. CBR-5884 inhibited PHGDH in a noncompetitive mode with respect to both substrates, as evidenced by a decreasing Vmax with increasing CBR-5884 concentration. The inhibition constants were 50 ± 20 μM and 50 ± 3 μM for 3-PG and NAD+, respectively (Fig. 4 A and B). We assessed whether there was any time dependence to the onset of inhibition by varying the time period for which drug and PHGDH were preincubated before initiating the enzymatic reaction. CBR-5884 was progressively more potent with increasing preincubation time, culminating in an IC50 of 7 μM when drug and PHGDH were preincubated for 4 h (Fig. 4C). Intrigued by the combination of a time-dependent onset of inhibition and noncompetitive inhibition, the latter suggesting that CBR-5884 might be binding to an allosteric pocket, we speculated that CBR-5884 could be affecting the PHGDH oligomerization state, where the time dependency of inhibition could potentially stem from drug-induced conformational changes in PHGDH. To evaluate the PHGDH oligomerization state, we incubated PHGDH with drug and then cross-linked before SDS/PAGE. CBR-5884 shifted the PHGDH equilibrium from the tetrameric to the dimeric state (Fig. 4D). No such effect was observed with LDH, which is resistant to CBR-5884–mediated inhibition (Fig. S3). CBR-5884 still inhibited a truncated form of PHGDH, which lacks the C-terminal domain responsible for tetramerization and is therefore a constitutive dimer (Fig. S3). Together, these results suggest that disruption of the tetramer might assist PHGDH inhibition but is not necessary for inhibition.
Fig. 4.
Mechanisms of CBR-5884 inhibition. Inhibition constants (Ki) were determined by titrating (A) 3-PG or (B) NAD+ while holding the other substrate constant at four different CBR-5884 concentrations and determining the initial reaction rate using a PHGDH assay. Plots were fit to a noncompetitive model. (C) Time-dependent inhibition was measured by preincubating drug and PHGDH for 0.5 h, 1 h, or 4 h as indicated before initiating the PHGDH reaction. Initial reaction rates (Vi) were determined and normalized to that of DMSO (Vo). (D) PHGDH was preincubated with CBR-5884 (0, 50, 200, or 400 μM) before cross-linking with BS3 (0.25 or 2.5 mM) followed by SDS/PAGE and Coomassie staining. Leftmost lane had no BS3 indicating the monomeric species. Oligomerization state was inferred from reference to a molecular weight ladder. Error is given as ±1 SD (n ≥ 3).
Fig. S3.
CBR-5884 does not affect the lactate dehydrogenase (LDH) oligomerization state but inhibits a truncated form of PHGDH. (A) LDH was preincubated with CBR-5884 (0, 50, 200, or 400 μM) for 30 min before cross-linking with BS3 (0.25 or 2.5 mM), followed by SDS/PAGE and Coomassie staining. Leftmost lane had no BS3, indicating the monomeric species. The oligomerization state was inferred from reference to a molecular weight ladder. (B) In vitro enzymatic assay using a truncated form of PHGDH (PHGDH3-314). Initial reaction rates (Vi) normalized to the DMSO control (Vo) are plotted. The IC50 for three independent experiments is given ±1 SD.
Discussion
We have reported the discovery of a PHGDH inhibitor, CBR-5884, and have shown that it inhibits serine synthesis in cells. Furthermore, CBR-5884 specifically inhibited the proliferation of melanoma and breast cancer lines with high levels of serine synthesis activity, with little effect on lines reliant on serine import. Thus, CBR-5884 is selective for lines addicted to serine synthesis and phenocopies sensitivity to PHGDH knockdown. Finally, a biochemical analysis of CBR-5884 revealed that it was a noncompetitive inhibitor that showed a time-dependent onset of inhibition and disrupted the oligomerization state of PHGDH.
Recent work examining how malignant cells rewire their metabolism to support growth and proliferation has revealed a number of clinically interesting targets (21, 22). Perhaps the most promising is the discovery of gain-of-function mutations in the isocitrate dehydrogenase (IDH) enzymes that result in the production of the oncometabolite 2-hydroxyglutarate (23, 24). The findings translated into chemical probes that yielded insights into the biology of IDH mutations (25, 26) and led to clinical programs (e.g., NCT02481154). The genetic evidence pointing to a role for PHGDH in cancer is similarly striking: PHGDH is one of few metabolic enzymes genetically deregulated in cancer (27, 28). Notably, elevated PHGDH expression correlates with clinical aggressiveness and poor prognosis in TNBC (13, 16) and NSCLC (15). There is a paucity of targeted therapies for these cancers and chemotherapies are frequently used (29, 30). Thus, the clinical potential of PHGDH inhibitors as targeted agents for TNBC and NSCLC tumors addicted to serine synthesis, as a single agent or in combination with standard of care, is an exciting perspective.
Beyond the preclinical applications, a PHGDH inhibitor provides a tool to study de novo serine synthesis. For example, it remains unclear why a serine biosynthesis enzyme is critical for tumor growth when serine is available in the serum (13, 14). CBR-5884 provides a valuable tool complementary to genetic strategies to study the necessity of serine synthesis and other phenomena because small molecules provide greater temporal resolution and do not deplete the actual protein. Moreover, CBR-5884 has already suggested an interesting feature of PHGDH biochemistry: namely, that human PHGDH could be regulated by transitions between different oligomerization states. These findings are reminiscent of pyruvate kinase M2 (PKM2) regulation in that both an endogenous metabolite, fructose-1,6-bisphosphate, and pharmacological small molecule activators enhance PKM2 activity by stabilizing the tetrameric form (31, 32). Endogenously, 2-phosphoglycerate (2-PG) has been reported to activate PHGDH (33). Mechanistically, 2-PG could be functioning by modulating the PHGDH oligomerization state. Finally, it is possible that CBR-5884 is a covalent inhibitor of PHGDH. Although PHGDH does not rely on an active site cysteine, there are a number of cysteines that could potentially perturb enzymatic function were they modified because they are in close proximity to the active site (PDB ID code 2G76). In an attempt to determine whether changes in the PHGDH oligomerization state are unique to CBR-5884, it would be interesting to determine whether Disulfiram (CBR-5807), CBR-6936, or sulfhydryl blocking reagents have similar effects.
The identification of a selective small molecule inhibitor of PHGDH capable of modulating de novo serine synthesis in PHGDH-dependent cancer cells represents a significant step toward the goal of targeting serine metabolism in oncology. However, future in vivo evaluation of the CBR-5884 chemical series will require medicinal chemistry-based optimization. Indeed, CBR-5884 was found to be unstable in mouse plasma, and, as described, replacement of the ethyl ester moiety with the corresponding negatively charged carboxylic acid resulted in a derivative that retains enzyme inhibitory activity but loses activity on cells. Thus, CBR-5884 is more likely to serve as a tool compound, and as a starting point for generating more drug-like molecules, than an actual drug. Furthermore, the cell-based potency of this series will likely need to be improved to enable in vivo evaluation at exposure levels that are not generally toxic. Nevertheless, our results provide a proof-of-concept that small molecule inhibitors of PHGDH represent a viable class of anti-cancer drugs.
Methods
Cells, Transfections, and Infections.
Breast and melanoma lines were passaged in RPMI supplemented with 10% (vol/vol) FBS, penicillin, streptomycin, and normocin (InvivoGen). Lentivirus was produced from Lenti-X 293T cells (Clontech) transfected with packaging plasmids pCMV-dR8.2 and pCMV-VSV-G and indicated pLKO.1 shRNAs: PHGDH, TRCN0000233029 (sh1) and TRCN0000221864 (sh2); nontargeting control, TRCN0000072181 (shGFP). Experiments were performed in compliance with Weill Cornell Medicine Environmental Health and Safety and Institutional Biosafety Committee. See SI Methods for details.
Immunoblots.
Protein was extracted from cells via trichloroacetic acid precipitation and blotted for with primary antibodies αPHGDH (HPA021241, 1/10,000; Sigma) and αVinculin (V9264, 1/5,000; Sigma). See SI Methods for details.
Proliferation Assays.
Cells were plated at a low density in 96- or 24-well plates in serine containing media. The following day, media were aspirated, cells were washed with PBS, and fresh serine-replete or -deplete media containing drug (15 μM, 30 μM) or vehicle (DMSO) were added. Cells were grown for 3–5 d, with drug and media changed daily before assaying relative cell numbers. See SI Methods for details.
Acute Drug Treatments with 13C6-Glucose Tracing.
Carney cells acclimated to growth in MEM (Corning) were plated at 9 × 105 cells per 6-cm dish the night before. The following morning, media were replaced with fresh media containing CBR-5884 (1 μM, 15 μM, 30 μM) or vehicle control (DMSO) for 1 h. Media were then aspirated, cells were washed with PBS, and fresh glucose-free MEM (Gibco) supplemented with 13C6-glucose (3 g/L; Cambridge Isotopes) and 10% (vol/vol) dialyzed FBS containing drug or DMSO was added. After 2 h, cells were quickly washed with cold PBS on ice and flash frozen. Polar metabolites were extracted as in the GCMS methods. See SI Methods for details.
Acute Toxicity Assay.
Carney cells acclimated to growth in MEM media were plated in a 96-well plate at 6,000 cells per well. The next day, cells were treated with CBR-5884 from 1 μM to 40 μM for 3 h. Drug containing media were then removed, fresh drug-free media added, and cell viability was determined via a CellTiter-Glo (G7572; Promega) or Alamar Blue (DAL1025; Invitrogen) assay according to the manufacturer’s protocol. See SI Methods for details.
GCMS Metabolite Analysis.
Polar metabolites were extracted with 2 mL of MeOH/H2O (4:1) for 30 min on dry ice, scraped, transferred to 2-mL tubes, and centrifuged (30 min, 21,000 × g), and the supernatants were dried under vacuum. Samples were derivatized as previously described (34) and analyzed on an Agilent 6890 GC instrument. Metabolite quantification was inferred from a standard curve, and fractional enrichment of 13C in metabolites was corrected for the natural abundance of 13C and 15N (35, 36). See SI Methods for details.
LC-MS/MS Metabolite Analysis.
Polar metabolites were extracted and dried as in the GCMS method. Samples were resuspended in 15 μL of HPLC grade water. Then, 5 μL of each sample was injected and analyzed using a 5500 QTRAP triple quadrupole mass spectrometer (AB/Sciex) coupled to a Prominence UFLC system (Shimadzu) as reported previously (37).
Protein Purification.
His6-tagged pET28a PHGDH, pET28a PSAT1, and pNIC28-Bsa4 PHGDH3-314 were purified via nickel agarose (Qiagen) from BL21 Escherichia coli cultures. pVB-CBD IDH1 was purified via Macroporous Bead Cellulose capture, TEV protease (Sigma-Aldrich) digestion, and gel filtration chromatography from BL21 E. coli cultures. See SI Methods for details.
PHGDH Assays.
PHGDH activity was measured in 96-well plates by monitoring NADH fluorescence [excitation wavelength (Ex) 340 nm/emission wavelength (Em) 460 nm] over time. PSAT1 was included to prevent product inhibition of PHGDH. See SI Methods for details.
LDH and MDH1 Assays.
Enzyme activities were assayed using kits (for LDH, MAK06, Sigma; for MDH1, MAK196-1KT, Sigma) according to the manufacturer’s instructions with commercially available recombinant enzyme (for LDH, 59747, Sigma; for MDH1, SRP6103, Sigma). Drug, titrated as for the PHGDH IC50 assays, and enzyme were preincubated for 30 min before initiating reaction with substrate.
Cross-Linking Assays.
PHGDH (1.5 μg) or LDH (2.2 μg, 59747; Sigma) was incubated with CBR-5884 (50 μM, 200 μM, 400 μM) or vehicle control (DMSO) in 25 mM Hepes, pH 7.3, and 1 mM NAD+ in 18 μL total volume for 30 min before BS3 (Pierce) cross-linking and quenching. Samples were run on SDS/PAGE and colloidal Coomassie stained (Bio-Rad). See SI Methods for details.
Primary PHGDH Screen, Diaphorase Counter Screen, and Dehydrogenase Panel Selectivity Profiling.
Compounds (800,000) were screened at a single dose (13.3 μM) in 1,536-plate format against PHGDH or diaphorase quantifying resorufin fluorescence (550/590 nm; Ex/Em) with an Envision plate reader. Results were analyzed using Genedata Screener software. Compounds with a robust Z-score of <−3 in the PHGDH screening assay and robust Z-score of >−2 in the diaphorase counter screen were selected as hits. See SI Methods for detailed protocols, hit selection and confirmation, and selectivity profiling against the dehydrogenase panel.
Chemical Syntheses.
CBR-5884, ethyl 5-(furan-2-carboxamido)-3-methyl-4-thiocyanatothiophene-2-carboxylate, and the acid derivative, 5-(furan-2-carboxamido)-3-methyl-4-thiocyanatothiophene-2-carboxylic acid, synthesis was adapted from the literature as described in SI Methods (38, 39).
SI Methods
Cells, Transfections, and Infections.
Breast and melanoma lines were passaged in RPMI supplemented with 10% FBS, penicillin, streptomycin, and normocin (InvivoGen). For lentivirus production and infection, Lenti-X 293T cells (Clontech) were transfected at 90% confluence with Lipofectamine 2000 (Invitrogen) with the indicated pLKO.1 shRNAs and packaging plasmids pCMV-dR8.2 and pCMV-VSV-G: for PHGDH, sh1, TRCN0000233029; sh2, TRCN0000221864; for nontargeting control, shGFP, TRCN0000072181. Viral supernatants were collected after 2 and 3 d. Filtered (0.45 μm) viral supernatant supplemented with 2 μg/mL polybrene was added to target cells overnight, and media changed the next morning. Selection (2 μg/mL puromycin) was initiated the following day for 3 d, at which point the cells were either harvested for protein or seeded for proliferation assays. Breast cell lines were obtained from ATCC, and melanoma lines were a gift from Haoqiang Ying (MD Anderson Cancer Center, Houston).
Immunoblots.
Media were removed, cells were washed with PBS, and proteins were isolated directly from intact cells via acid extraction using a 10% TCA solution (10 mM Tris⋅HCl, pH 8.0, 10% trichloroacetic acid, 25 mM NH4OAc, 1 mM Na2EDTA). Precipitated proteins were harvested and solubilized in a 0.1 M Tris⋅HCl, pH 11, solution containing 3% SDS and boiled for 5–10 min. Lysate protein content was quantified via the BCA method, run on an SDS/PAGE page, transferred to a nitrocellulose membrane, blocked (5% BSA), and probed with primary antibodies αPHGDH (HPA021241, 1/10,000, 5% BSA; Sigma) and αVinculin (V9264, 1/5,000, 5% BSA; Sigma).
Proliferation Assays.
Cells were plated at a low density in 96- or 24-well plates in serine-containing media. The following day, media were aspirated, cells were washed with PBS, and fresh serine-replete or serine-deplete media containing drug (15 μM, 30 μM) or vehicle (DMSO) were added. Serine-replete or -deplete media were made from serine/glycine-free DMEM (containing pyruvate) that had been supplemented with 10% dialyzed FBS and either serine (400 μM final, serine-replete) or PBS (serine-deplete). Cells were grown for 3–5 d, adding fresh drug containing media everyday, before assaying relative cell numbers. For 96-well plates, relative cell numbers were determined using a Cyquant (Life Technologies) assay according to the manufacturer’s instructions. For 24-well plates, crystal violet staining was performed as follows: Cells were washed with PBS, fixed with 10% formalin (10 min), washed twice with PBS, and stored at 4 °C in PBS until the completion of the experiment, after which PBS was removed and cells were stained with 0.1% crystal violet in 20% methanol for 15 min. After staining, cells were washed three times with water and air dried overnight. Cell-bound crystal violet was solubilized in 10% acetic acid and 595-nm absorbance was measured. Serine/glycine-free DMEM was custom ordered from Gibco.
Carney Serine Labeling Time Course and Acute Drug Treatments with 13C6-Glucose Tracing.
Carney cells were acclimated to growth in MEM (Corning) supplemented with 10% dialyzed FBS, penicillin, streptomycin, and normocin (InvivoGen) by passaging for 2 wk (approximately six passages). Cells were plated at 9 × 105 cells per 6-cm dish 1 d before the experiment. For the serine-labeling time course, cells were washed with PBS and fresh glucose-free MEM supplemented with 13C6-glucose (3 g/L; Cambridge Isotopes) and 10% dialyzed FBS was added. Cells were then incubated at 37 °C and harvested, over nine time points covering a range of 0.12 h to 6 h, by quickly washing cells with cold PBS and flash freezing. For acute drug inhibition assays, media was replaced with fresh media containing CBR-5884 (1 μM, 15 μM, 30 μM) or vehicle control (DMSO) for 1 h. Media were then aspirated, cells were washed with PBS, and fresh glucose-free MEM supplemented with 13C6-glucose (3g/L; Cambridge Isotopes) and 10% dialyzed FBS containing drug or DMSO was added. After 2 h, cells were quickly washed with cold PBS on ice and flash frozen. Polar metabolites were then extracted and analyzed as described in the GCMS methods. The seven initial lead PHGDH inhibitors were assayed as in the CBR-5884 dose–response experiment, except that only 30 μM drug or DMSO control conditions were used. Glucose-free MEM was custom ordered from Gibco.
Acute Toxicity Assay.
Carney cells acclimated to growth in MEM media supplemented with 10% dialyzed FBS, penicillin, streptomycin, and normocin (InvivoGen) were plated in a 96-well plate at 6,000 cells per well. The next day, cells were treated with CBR-5884 from 1 μM to 40 μM for 3 h. Drug-containing media was then removed, fresh drug-free media added, and cell viability was determined via a CellTiter-Glo (G7572; Promega) or Alamar Blue (DAL1025; Invitrogen) assay according to the manufacturers’ protocol. For Alamar Blue assays, cells were incubated with Alamar Blue-containing media for 2 h at 37 °C after removal of drug before reading fluorescence.
GCMS Metabolite Analysis.
Polar metabolites were extracted with 2 mL of MeOH/H2O (4:1) for 30 min on dry ice, scraped, transferred to 2-mL tubes, and centrifuged (30 min, 21,000 × g), and the supernatants were dried under vacuum. Samples were derivatized as previously described using methoxyamine hydrochloride and N-methyl-N-tert-butyldimethylsilyltrifluoroacetamide (34). Samples were pulse spun to remove insoluble matter, and the supernatant was transferred to inserts set in brown glass vials and capped. Analysis was performed on an Agilent 6890 GC instrument. Samples were loaded onto a 30-m DB-35MS capillary column using helium carrier gas and interfaced to an Agilent 5975B MS. Electron impact (EI) ionization was set at 70 eV. Each sample was injected at 270 °C at a flow rate of 1 mL/min. To mobilize metabolites, the GC oven temperature was held at 100 °C for 3 min and increased to 300 °C at 3.5 °C/min for a total run time of ∼1 h. Then, 1 μL of each sample was injected in splitless mode. All analyses were operated in full scan mode while recording mass-to-charge ratio (Δm/z) spectra in the range of 100–650 m/z. Specific metabolite Δm/z analyzed are available upon request. For quantification of metabolites, unknown samples were fit to a standard curve of known metabolites assessed in the linear range of 0.006–0.33 nmol on column. Standards were run in parallel to samples to maintain accuracy of retrofit quantitation. Fractional enrichment of 13C in metabolites was corrected for the natural abundance of 13C and 15N using METRAN and in-house scripts written in Matlab (35, 36).
PHGDH and PSAT1 Purification.
pET28a PHGDH, pET28a PSAT1, or pNIC28-Bsa4 PHGDH3-314 was transformed into BL21 Escherichia coli. A single colony was grown to an OD600 0.7 in 1 L of Luria broth, and protein expression was induced with 0.5 mM isopropyl thiogalactopyranoside (IPTG). The culture was chilled on ice for 30 min, cultured for 20 h at room temp, pelleted (6,000 g, 20 min), and flash frozen. Pellets were resuspended in 60 mL of lysis buffer (50 mM Tris, pH 8.5, 10 mM MgCl2, 300 mM NaCl, 10% glycerol, 5 mM imidazole) and sonicated, and cell debris was pelleted by centrifugation (20,000 × g, 30 min). The supernatant was collected, and 2 mL of Ni-agarose beads (Qiagen), prewashed twice with wash buffer (50 mM Tris, pH 8.5, 10 mM MgCl2, 300 mM NaCl, 10% glycerol, 30 mM imidazole), and 60 μL of 2-mercaptoethanol were added. Beads and lysate were incubated for 3 h on a rocker at 4 °C. Beads were batch-washed four times with wash buffer and transferred to a column. Bound proteins were eluted (elution buffer: 50 mM Tris, pH 8.5, 10 mM MgCl2, 250 mM NaCl, 10% glycerol, 250 mM imidazole), collecting 1-mL fractions. Fraction protein content was measured via a Bradford assay. The most concentrated fractions were pooled and dialyzed overnight into 2 L of dialysis buffer (50 mM Tris, pH 8.5, 10 mM MgCl2, 250 mM NaCl, 20% glycerol, 0.15% 2-mercaptoethanol). The next morning, dialysis buffer was changed, and dialysis was continued for 4 h. Protein purity was assessed via SDS/PAGE and Coomassie staining.
IDH1 Purification.
The IDH1 gene was PCR amplified from SC322129 [IDH1 (NM_005896) Human cDNA] from Origene. The cDNA sequence was cloned into a pVB-CBD vector by inserting the IDH1 gene downstream of OmpA and cellulose binding domain (CBD). BL21-Gold (DE3) cells (Agilent) were transformed with the pVB-CBD-IDH1 plasmid, and single colonies were generated. Protein expression was performed under IPTG (1 mM) induction at 25 °C for 24 h. The supernatant containing the IDH1 protein was separated by centrifugation. The IDH1-CBD was captured on a Macroporous Bead Cellulose MT100 column (Iontosorb). TEV protease (Sigma-Aldrich) digestion was performed at 37 °C for 1 h. Size exclusion FPLC was performed using a Superdex 200 10/300 GL (GE Health Care Life Sciences) column. Purified protein purity was more than 95% as determined by SDS/PAGE.
PHGDH Assays.
PHGDH activity was measured in 96-well plates (100 μL per well) at 28 °C by monitoring NADH fluorescence (Ex 340 nM/Em 460 nM) over time with a FLUOstar Omega (BMG Labtech). PSAT1 was included to prevent product inhibition of PHGDH. Assays were performed in PHGDH assay buffer (50 mM Tris, pH 8.5, and 1 mM EDTA). Substrates and enzyme concentrations were as follows: 3-phosphoglycerate, 240 μM; NAD+, 120 μM; glutamate, 30 mM; 5.7 ng/μL for full-length PHGDH or 240 ng/μL for PHGDH3-314; PSAT1, 80 ng/μL, except for Ki measurements where one of the PHGDH substrates was held constant at 3 mM whereas the other substrate was titrated from 2 mM to 8 μM for NAD+ or 1 mM to 0.15 μM for 3-PG. An NADH standard curve in PHGDH assay buffer was included to quantify fluorescent signal. For IC50 assays, a twofold serial dilution of drug ranging from 160 μM to 0.15 μM was preincubated with enzyme for 30 min before initiating the enzyme reaction with substrate mix. For time-dependent IC50 assays, drug preincubation time was 4 h, 1 h, or 30 min. For Ki measurements, drug (0 μM, 15 μM, 30 μM, 80 μM) and enzyme were preincubated for 30 min. Initial rate plots were fit using Prism. Chemicals were purchased from Sigma.
Cross-Linking Assays.
PHGDH (1.5 μg) or LDH (2.2 μg, 59747; Sigma) was incubated with CBR-5884 (50 μM, 200 μM, 400 μM) or vehicle control (DMSO) in 25 mM Hepes, pH 7.3, and 1 mM NAD+ in 18 μL total volume for 30 min. BS3 (PI-21585; Pierce) cross-linker dissolved in PBS was added to a final concentration of 0 mM, 0.25 mM, or 2.5 mM and incubated for 30 min on a rocker. The reaction was then quenched for 15 min by adding 1 M Tris, pH 7.5, to a final concentration of 27 mM. Cross-linked proteins were mixed with sample buffer, boiled for 5 min, and run on SDS/PAGE. Gels were stained with colloidal Coomassie stain (161-0803; Bio-Rad) for 24 h and destained according to the manufacturer’s instructions.
Primary PHGDH Screen.
Compounds (800,000) were screened at a single dose (13.3 μM) in 1,536-plate format. Bromopyruvate (0.5 mM) was used as inhibitor control. For assay setup, 2 μL of assay buffer was added to each well of a 1,536-well plate. Compounds were transferred, and 2 μL of reaction mixture 2 was added followed by a 3-min incubation. Reactions were started by adding 2 μL of reaction mixture 1. Plates were incubated (30 min at 37 °C) before fluorescence quantification (550/590 nm, Ex/Em) using an Envision plate reader. Reaction mixture 1 was as follows: 3-phosphoglycerate (0.25 mM), resazurin (0.1 mM), NAD+ (8.3 mM), glutamic acid (30 mM), Tris⋅HCl (30 mM), pH 8.0, and 0.6 mM EDTA. Reaction mixture 2 was as follows: PSAT1 (0.02 μg/μL), PHGDH (0.005 μg/μL), and diaphorase (0.001 U/μL) diluted in Tris⋅HCl (30 mM), pH 8.0, and EDTA (0.6 mM) buffer. Assay buffer was as follows: Tris⋅HCl, pH 8.0 (30 mM) and EDTA (0.6 mM).
Diaphorase Counter Screen.
All 800,000 compounds were screened against diaphorase (Worthington Biochemical Corporation) as in the primary screen, but drug and enzyme were incubated for 20 min at 25 °C before initiating the reaction. Modified reaction mixtures were as follows: mixture 1, resazurin (0.1 mM), NADPH (0.1 mM), and buffer (30 mM Tris⋅HCl, 0.6 mM EDTA, pH 8.0); mixture 2, diaphorase (0.0002 U/μL), buffer (30 mM Tris⋅HCl, 0.6 mM EDTA, pH 8.0).
Screening Hit Selection Criteria, Confirmation, and Selectivity Profiling.
Results were analyzed using Genedata Screener software. Compounds with a robust Z-score of <−3 in the PHGDH screening assay and robust Z-score of >−2 in the diaphorase counter screen were selected as hits. Hit confirmation was done in triplicate (13.3 μM) in 1,536-plate format as in the primary PHGDH screen. To assess selectivity of confirmed hits, hits were tested in 1,536-plate format in an eight-point dose–response experiment (twofold serial dilution) against a panel of dehydrogenases including the following: PHGDH, isocitrate dehydrogenase (IDH1), malate dehydrogenase (MDH1), and 3α-hydroxysteroid dehydrogenase (3α-HSD). The PHGDH assay was performed as in the primary screen. For the IDH1 assay, 2 µL of assay buffer was added to each well. Compounds were transferred and 2 µL of reaction mixture 2 was added, followed by a 3-min incubation. Reactions were started by the addition of 2 µL of reaction mixture 1. Plate was incubated for 45 min at 37 °C before fluorescence quantification. For the MDH1 assay, 2 µL of assay buffer was added to each well. Compounds were transferred, and 2 µL of reaction mixture 4 was added, followed by a 3-min incubation. Reactions were started by the addition of 2 µL of reaction mixture 3. Plate was incubated for 2.5 h at 37 °C before fluorescence quantification. For the 3α-HSD assay, 2 µL of assay buffer was added to each well. Compounds were transferred, and 2 μL of reaction mixture 6 was added, followed by a 3-min incubation. Reactions were started by the addition of 2 µL of reaction mixture 5. Plate was incubated for 30 min at 37 °C before fluorescence quantification. In all cases, fluorescence (550/590 nm; Ex/Em) was quantified with an Envision plate reader, and IC50 values were obtained using Genedata Screener software. Compounds that were not at least fourfold selective for PHGDH were eliminated, and the seven most potent PHGDH inhibitors were selected for further cell-based work. Reaction mixture 1 was as follows: isocitrate (0.36 mM), resazurin (0.1 mM), NADP+ (0.033 mM), MnCl2 (1 mM), and buffer (30 mM Tris⋅HCl, 0.6 mM EDTA, pH 8.0). Reaction mixture 2 was as follows: IDH1 (0.25 µg/µL; purified in house) and diaphorase (0.005 U/µL) diluted in buffer (30 mM Tris⋅HCl, 0.6 mM EDTA, pH 8.0). Reaction mixture 3 was as follows: l-malic acid (2 mM), resazurin (0.1 mM), NAD+ (0.5 mM), and buffer (30 mM Tris⋅HCl, 0.6 mM EDTA, pH 8.0). Reaction mixture 4 was as follows: porcine MDH1 (M2634, 0.00025 µg/µL; Sigma), diaphorase (0.005 U/µL), and buffer (30 mM Tris⋅HCl, 0.6 mM EDTA, pH 8.0). Reaction mixture 5 was as follows: cholic acid (0.05 mM), resazurin (0.1 mM), NAD+ (0.007 mM), and buffer (20 mM sodium pyrophosphate, pH 9.0, 4 µg/mL BSA). Reaction mixture 6 was as follows: 3α-HSD (0.025 µg/µL; Sigma), diaphorase (0.005 U/µL), and buffer (20 mM sodium pyrophosphate, pH 9.0, 4 µg/mL BSA). Chemicals were purchased from Sigma and diaphorase from Worthington Biochemical Corporation.
SI Chemical Syntheses
Materials.
The 2-amino-4-methyl-3-cyanato-5-thiophenecarboxylate ethyl ester was obtained from Life Chemicals. Cyanoacetic acid was obtained from AK Scientific. All remaining reagents and solvents were obtained from Sigma-Aldrich. All reagents were used as received without further purification.
Ethyl 5-(Furan-2-Carboxamido)-3-Methyl-4-Thiocyanatothiophene-2-Carboxylate.
The 86.3 mg of 2-amino-4-methyl-3-cyanato-5-thiophenecarboxylate ethyl ester (357 µmol, 1 eq) was suspended
 |
in 1 mL of dichloromethane (DCM) with a magnetic stir bar. Then, 86.5 µL of pyridine (1,070 µmol, 3 eq) was added to the mixture, followed by drop-wise addition of 35.3 µL of 2-furoyl chloride (357 µmol, 1 eq). After 1 h, the reaction mixture was purified directly by normal-phase silica gel chromatography. Collected fractions were dried in vacuo to obtain 87.2 mg of ethyl 5-(furan-2-carboxamido)-3-methyl-4-thiocyanatothiophene-2-carboxylate as a white solid, 73% yield. 1H NMR (400 MHz CDCl3) δ 9.36 (s, 1H), 7.67 (dd, J = 0.8, 1.7 Hz, 1H), 7.41 (dd, J = 0.8, 3.6 Hz, 1H), 6.67 (dd, J = 1.7, 3.6 Hz, 1H), 4.36 (q, J = 7.1 Hz, 2H), 1.40 (t, J = 7.1 Hz, 3H) ppm. LC-MS (M+H)+ = 337.09, (M-CN)+ = 310.09.
Tert-Butyl 4-Cyano-3-Methylbut-3-Enoate.
The following was adapted from a procedure in ref. 39: 5.00 g (31.6 mmol, 1 eq) of tert-butyl acetoacetate, 2.69 g (31.6 mmol, 1 eq) of cyanoacetic acid, and 488 mg (6.33 mmol, 0.2 eq) of ammonium acetate were added to a solution of 0.9 mL of acetic acid and 9.0 mL of benzene. The reaction mixture was refluxed in a Dean-Stark trap for 24 h. After cooling to room temperature, the reaction mixture was washed with saturated sodium bicarbonate and brine, and then dried with sodium sulfate and condensed in vacuo. The crude product was vacuum-distilled at 65 °C to obtain 1.28 g of tert-butyl 4-cyano-3-methylbut-3-enoate as a mixture of E/Z isomers, 23% yield, clear oil. 1H NMR (400 MHz CDCl3) δ 5.30 (m, 2H), 3.36 (d, J = 1.5 Hz, 1H), 3.11 (d, J = 1.1 Hz, 1H), 2.15 (d, J = 1.1 Hz, 1H), 2.02 (d, J = 1.5 Hz, 1H), 1.48 (m, 18H) ppm.
 |
Tert-Butyl 5-Amino-3-Methylthiophene-2-Carboxylate.
The following was adapted from a procedure in ref. 39: 1.28 g (7.09 mmol, 1 eq) of tert-butyl 4-cyano-3-methylbut-3-enoate and 227 mg (7.09 mmol, 1 eq) of sulfur flakes were suspended in 5.5 mL of ethanol. Then, 806 µL (7.80 mmol, 1.1 eq) of diethylamine was added drop-wise, and the mixture was stirred for 4 h, forming a dark orange/red solution. The reaction mixture was dried in vacuo and purified by flash chromatography. After drying collected fractions in vacuo, 814 mg of tert-butyl 5-amino-3-methylthiophene-2-carboxylate was obtained as a yellow-orange oil, 54% yield. 1H NMR (400 MHz CDCl3) δ 5.99 (s, 1H), 4.01 (br. s, 2H), 2.40 (s, 3H), 1.552 (s, 9H) ppm.
Tert-Butyl 5-Amino-3-Methyl-4-Thiocyanatothiophene-2-Carboxylate.
One hundred milligrams (0.469 mmol, 1 eq) of tert-butyl 5-amino-3-methylthiophene-2-carboxylate and 71.4 mg (0.938 mmol, 2 eq) of ammonium thiocyanate were dissolved in 0.5 mL of methanol and cooled with an ice bath. A solution of 14.9 µL (0.291 mmol, 0.62 eq) of bromine in methanol was added drop-wise to the reaction mixture. The mixture was stirred on ice for 10 min; then, the ice bath was removed and the mixture was stirred for another hour while warming to room temperature. The reaction mixture was then dried in vacuo and purified by flash chromatography. The collected fractions were dried in vacuo to afford 32.0 mg of tert-butyl 5-amino-3-methyl-4-thiocyanatothiophene-2-carboxylate as a white solid. 1H NMR (400 MHz CDCl3) δ 5.05 (br. s, 1H), 2.59 (2, 3H), 1.57 (s, 9H) ppm.
Tert-Butyl 5-(Furan-2-Carboxamido)-3-Methyl-4-Thiocyanatothiophene-2-Carboxylate.
The following was prepared analogously to the ethyl 5-(furan-2-carboxamido)-3-methyl-4-thiocyanatothiophene-2-carboxylate derivative. From 32.0 mg (0.119 mmol) of tert-butyl 5-amino-3-methyl-4-thiocyanatothiophene-2-carboxylate as starting material, 19.8 mg of tert-butyl 5-(furan-2-carboxamido)-3-methyl-4-thiocyanatothiophene-2-carboxylate was obtained as a white solid, 46% yield. 1H NMR (400 MHz CDCl3) δ 9.35 (s 1H), 7.68 (dd, J = 0.8, 1.7 Hz, 1H), 7.42 (dd, J = 0.8, 3.6 Hz, 1H), 6.68 (dd, J = 1.8, 3.6 Hz, 1H), 2.72 (s, 3H), 1.60 (s, 9H) ppm.
5-(Furan-2-Carboxamido)-3-Methyl-4-Thiocyanatothiophene-2-Carboxylic Acid.
Trifluoroacetic acid (0.25 mL) was added slowly to a solution of 5.3 mg (13.7 µmol) of tert-butyl 5-(furan-2-carboxamido)-3-methyl-4-thiocyanatothiophene-2-carboxylate in 0.25 mL of dichloromethane in an ice bath and stirred for 30 min, allowing the mixture to warm to room temperature over time. The reaction mixture was dried in vacuo with minimal heating applied (no greater than 30 °C). The dried product was sonicated in diethyl ether to dissolve the remaining starting material, centrifuged briefly to precipitate the product, and then decanted to remove the starting material in the ether layer. This ether treatment was repeated two more times. After drying the remaining solid in vacuo, 1.6 mg of 5-(furan-2-carboxamido)-3-methyl-4-thiocyanatothiophene-2-carboxylic acid was obtained as a white solid, 38% yield. 1H NMR (400 MHz CDCl3) δ 7.89 (dd, J = 0.8, 1.8 Hz, 1H), 7.46 (dd, J = 0.8, 3.6 Hz, 1H), 6.75 (dd, J = 1.8, 3.6 Hz, 1H), 2.71 (s, 3H) ppm. LC-MS (M+H)+ = 309.0, (M-OH)+ = 291.0, (M-CN)+ = 282.0.
Supplementary Material
Acknowledgments
We thank U. Oppermann, M. G. Vander Heiden, K. R. Mattaini, and M. Yuan for technical assistance and reagents. We thank J. Johnson, Y. Zheng, H. Shim, B. D. Ngo, and other L.C.C. laboratory members for helpful discussions. L.C.C. was supported by NIH Grants P01CA117969 and P01CA120964. C.A.L. was partially supported by a PanCAN-AACR Pathway to Leadership Award and a Dale F. Frey Award for Breakthrough Scientists from the Damon Runyon Cancer Research Foundation (Grant DFS-09-14). G.M.D. was supported by a PanCAN-AACR Pathway to Leadership Award. S.-M.F. was supported by a Conquer Cancer Now Award from the Concern Foundation.
Footnotes
Conflict of interest statement: L.C.C. owns equity in, receives compensation from, and serves on the Board of Directors and Scientific Advisory Board of Agios Pharmaceuticals. Agios Pharmaceuticals is identifying metabolic pathways of cancer cells and developing drugs to inhibit such enzymes to disrupt tumor cell growth and survival.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1521548113/-/DCSupplemental.
References
- 1.Kuge O, Hasegawa K, Saito K, Nishijima M. Control of phosphatidylserine biosynthesis through phosphatidylserine-mediated inhibition of phosphatidylserine synthase I in Chinese hamster ovary cells. Proc Natl Acad Sci USA. 1998;95(8):4199–4203. doi: 10.1073/pnas.95.8.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Koning TJ, et al. L-serine in disease and development. Biochem J. 2003;371(Pt 3):653–661. doi: 10.1042/BJ20021785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Futerman AH, Riezman H. The ins and outs of sphingolipid synthesis. Trends Cell Biol. 2005;15(6):312–318. doi: 10.1016/j.tcb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Stover P, Schirch V. Serine hydroxymethyltransferase catalyzes the hydrolysis of 5,10-methenyltetrahydrofolate to 5-formyltetrahydrofolate. J Biol Chem. 1990;265(24):14227–14233. [PubMed] [Google Scholar]
- 5.Tibbetts AS, Appling DR. Compartmentalization of mammalian folate-mediated one-carbon metabolism. Annu Rev Nutr. 2010;30(1):57–81. doi: 10.1146/annurev.nutr.012809.104810. [DOI] [PubMed] [Google Scholar]
- 6.Palacín M, Estévez R, Bertran J, Zorzano A. Molecular biology of mammalian plasma membrane amino acid transporters. Physiol Rev. 1998;78(4):969–1054. doi: 10.1152/physrev.1998.78.4.969. [DOI] [PubMed] [Google Scholar]
- 7.Barker GA, Ellory JC. The identification of neutral amino acid transport systems. Exp Physiol. 1990;75(1):3–26. doi: 10.1113/expphysiol.1990.sp003382. [DOI] [PubMed] [Google Scholar]
- 8.Snell K. The duality of pathways for serine biosynthesis is a fallacy. Trends Biochem Sci. 1986;11(6):241–243. [Google Scholar]
- 9.Mullarky E, Mattaini KR, Vander Heiden MG, Cantley LC, Locasale JW. PHGDH amplification and altered glucose metabolism in human melanoma. Pigment Cell Melanoma Res. 2011;24(6):1112–1115. doi: 10.1111/j.1755-148X.2011.00919.x. [DOI] [PubMed] [Google Scholar]
- 10.Snell K, Natsumeda Y, Eble JN, Glover JL, Weber G. Enzymic imbalance in serine metabolism in human colon carcinoma and rat sarcoma. Br J Cancer. 1988;57(1):87–90. doi: 10.1038/bjc.1988.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snell K, Natsumeda Y, Weber G. The modulation of serine metabolism in hepatoma 3924A during different phases of cellular proliferation in culture. Biochem J. 1987;245(2):609–612. doi: 10.1042/bj2450609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beroukhim R, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463(7283):899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Locasale JW, et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet. 2011;43(9):869–874. doi: 10.1038/ng.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Possemato R, et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476(7360):346–350. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeNicola GM, et al. NRF2 regulates serine biosynthesis in non-small cell lung cancer. Nat Genet. 2015;47(12):1475–1481. doi: 10.1038/ng.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollari S, et al. Enhanced serine production by bone metastatic breast cancer cells stimulates osteoclastogenesis. Breast Cancer Res Treat. 2011;125(2):421–430. doi: 10.1007/s10549-010-0848-5. [DOI] [PubMed] [Google Scholar]
- 17.Zhang JH, Chung TD, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4(2):67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 18.Vallari RC, Pietruszko R. Human aldehyde dehydrogenase: Mechanism of inhibition of disulfiram. Science. 1982;216(4546):637–639. doi: 10.1126/science.7071604. [DOI] [PubMed] [Google Scholar]
- 19.Buescher JM, et al. A roadmap for interpreting (13)C metabolite labeling patterns from cells. Curr Opin Biotechnol. 2015;34:189–201. doi: 10.1016/j.copbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barretina J, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483(7391):603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Son J, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496(7443):101–105. doi: 10.1038/nature12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tennant DA, Durán RV, Gottlieb E. Targeting metabolic transformation for cancer therapy. Nat Rev Cancer. 2010;10(4):267–277. doi: 10.1038/nrc2817. [DOI] [PubMed] [Google Scholar]
- 23.Dang L, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462(7274):739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parsons DW, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang F, et al. Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation. Science. 2013;340(6132):622–626. doi: 10.1126/science.1234769. [DOI] [PubMed] [Google Scholar]
- 26.Rohle D, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340(6132):626–630. doi: 10.1126/science.1236062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mullen AR, DeBerardinis RJ. Genetically-defined metabolic reprogramming in cancer. Trends Endocrinol Metab. 2012;23(11):552–559. doi: 10.1016/j.tem.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gottlieb E, Tomlinson IPM. Mitochondrial tumour suppressors: A genetic and biochemical update. Nat Rev Cancer. 2005;5(11):857–866. doi: 10.1038/nrc1737. [DOI] [PubMed] [Google Scholar]
- 29.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 30.Boolell V, Alamgeer M, Watkins DN, Ganju V. The evolution of therapies in non-small cell lung cancer. Cancers (Basel) 2015;7(3):1815–1846. doi: 10.3390/cancers7030864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anastasiou D, et al. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat Chem Biol. 2012;8(10):839–847. doi: 10.1038/nchembio.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kung C, et al. Small molecule activation of PKM2 in cancer cells induces serine auxotrophy. Chem Biol. 2012;19(9):1187–1198. doi: 10.1016/j.chembiol.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hitosugi T, et al. Phosphoglycerate mutase 1 coordinates glycolysis and biosynthesis to promote tumor growth. Cancer Cell. 2012;22(5):585–600. doi: 10.1016/j.ccr.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicolay BN, et al. Loss of RBF1 changes glutamine catabolism. Genes Dev. 2013;27(2):182–196. doi: 10.1101/gad.206227.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antoniewicz MR, Kelleher JK, Stephanopoulos G. Determination of confidence intervals of metabolic fluxes estimated from stable isotope measurements. Metab Eng. 2006;8(4):324–337. doi: 10.1016/j.ymben.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Antoniewicz MR, Kelleher JK, Stephanopoulos G. Elementary metabolite units (EMU): A novel framework for modeling isotopic distributions. Metab Eng. 2007;9(1):68–86. doi: 10.1016/j.ymben.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ying H, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149(3):656–670. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guzi JT, et al. (2007) Methods for inhibiting protein kinases. US Patent Appl 11/598,188:1–346.
- 39.Ambrogi V, Grandolini G, Perioli L, Rossi C. Convenient synthesis of 2-aminonaphthalene-1-thiol and 3-aminoquinoline-4-thiol and cyclocondensations to 1,4-thiazino and 1,4-thiazepino derivatives. Synthesis. 1992;1992(7):656–658. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.