Abstract
The Fischer 344/NNiaHSD × Brown Norway/BiNia F1 (F344xBN) rat model exhibits an increased life span and fewer age-associated pathologies compared to commonly used Fischer 344 (F344). How aging may affect cardiac structure and function in these animals, has to our knowledge, not been investigated. Echocardiography was performed on female F344xBN rats at 6, 26, and 30 months of age using a Phillips 5500 Echocardiography system. Before sacrifice, electrocardiograms were measured in the female F344xBN in order to determine heart rhythm interval changes. Aging was associated with an increase in heart to body weight ratio, cardiomyocyte cross-sectional area, posterior wall thickening, and left ventricle chamber dilatation. Aging was associated with slight evidence of diastolic dysfunction. Alterations in heart rhythm intervals were associated with alterations in the spatial distribution of connexin 43. The incidence of arrhythmias was not different with age; however, valvular dysfunction was increased. These data suggest that aging in the female F344xBN rat heart is associated with changes in cardiac structure as well as function. Further investigation regarding other parameters of cardiac biochemistry and function is needed to better understand the normal compensated cardiovascular aging process in the female F344xBN.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-014-9684-6) contains supplementary material, which is available to authorized users.
Keywords: Aging, Female, Echocardiography, Arrythmias, Connexin 43
Introduction
Aging is associated with an increased risk of developing myocardial infarction, stroke, atherosclerosis, peripheral occlusive disease, diabetes, and hypertension (Sohal and Weindruch 1996; Finkel and Holbrook 2000). Similar to that seen in humans, aging in rodents is characterized by cardiomyocyte loss, hypertrophy of the remaining cardiomyocytes, and increased fibrosis (Lakatta 2003; Anversa et al. 1990; Betsuyaku et al. 2002; Rossi et al. 2008). These structural changes adversely affect aging cardiac function by prolonging contraction and relaxation leading to systolic and diastolic dysfunction (Lakatta 1998; Hacker et al. 2006).
The Fischer 344 × Brown Norway F1 (F344xBN) has been recommended by the National Institute of Aging for aging studies due to its increased life span and the presence of fewer age-associated pathologies when compared to other rodent models (Lipman et al. 1996; Turturro et al. 1999). It has been demonstrated that male F344xBN animals undergo similar age-associated changes in cardiac structure and function to those seen in humans. Whether female F344xBN animals exhibit similar alterations has, to our knowledge, not been examined.
Normal cardiovascular aging can lead to structural changes in addition to decreased connexin 43 (Cx43) expression or increased heterogeneity (Lancaster et al. 2011) which can lead to electrical abnormalities by disrupting normal myofiber organization as well as slowing conductance (Kawara et al. 2001). Such changes in electrical conductance, electrolyte imbalance, and altered ion channels have been linked to various forms of cardiac disease and rhythm disturbances (Rossi et al. 2008; El-Sherif and Turitto 2011; Nattel et al. 2007). These adaptations are capable of resulting in secondary cardiac dysfunction, which can include alterations in cardiac rhythm. To our knowledge such changes have not been investigated in the female F344xBN. These changes, if present, in a non-pathological model (Lipman et al. 1996) of female aging may provide insight into the normal compensated cardiovascular aging process within the female heart.
The purpose of this study was to examine how aging affects the structure and function of the female F344xBN rat heart and to determine if changes in tissue structure and function, if present, are associated with changes in electrocardiographic measures and Cx43 heterogeneity of distribution. We hypothesize that alterations in cardiac structure and Cx43 heterogeneity distribution in the female F344xBN heart will be associated with cardiac dysfunction and heart rhythm interval changes.
Materials and methods
Animals
All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals as approved by the Council of the American Physiological Society and the Animal Use Review Board of Marshall University. All procedures were conducted in strict accordance with the Public Health Service Animal Welfare Policy. Virgin adult (6-month), aged (26-month), and very aged (30-month) female Fischer 344/NNiaHSd × Brown Norway/BiNia F1 hybrid (F344xBN) rats were obtained from the National Institute for Aging and housed two per cage in an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) approved vivarium. Animal ages were chosen based on survivability curves from the National Institute of Aging to approximate females in the third, seventh, and eighth decades of life. Given that previous data have demonstrated a complete loss of cyclicity at 16 months of age, the estrous phase was not monitored (PMID 17460359). Housing conditions consisted of a 12 h light–12 h dark cycle and temperature was maintained at 22 ± 2 °C. Animals were provided with food and water ad libitum. Rats were allowed to recover from shipment for at least 2 weeks before experimentation during which time the animals were carefully observed and weighed weekly. Any of the rats showing signs of failure to thrive, such as precipitous weight loss, disinterest in environment, or unexpected gait alterations were excluded from the study.
Echocardiographic procedures
Rats (6, 26, and 30 months) were anesthetized using a cocktail of ketamine (40 mg/kg) and xylazine (10 kg/mg) which was injected into the intraperitoneal cavity in order to perform echocardiograms. Echocardiographic procedures were done as previously described by Walker and colleagues (Walker et al. 2006). In order to prevent disturbances of ultrasound waves the ventral thorax was shaved and the rats were placed either on their backs or left side and covered with ultrasonic transmission gel. A Phillips 5500 Echocardiographic system with a 12-MHz transducer was used to take two-dimensional echocardiographic measurements, two-dimensional guided M-mode, Doppler M-mode, and parasternal long- and shot-axis views. Parasternal long- and short-axis views were used to determine two-dimensional cardiac structural measurements. The echocardiographic views were then used to position the M-mode echocardiographic line. Valvular blood flow velocities were evaluated using pulse wave Doppler with the probe toward the apex (x-axis) and the depth along the y-axis (long axis procedure). Positioning the probe toward the left ventricle and across the heart made during short axis procedures made it possible to evaluate wall structure in order to calculate ejection fraction and fractional shortening during systole. A digital echocardiographic analysis system was used to analyze M-mode displays. All echocardiography procedures and parameters were measured by the same echocardiogram technician to limit inter-observer variability.
Left ventricular mass (LVM) was calculated according to the following equation on the basis of previous reports demonstrating a good correlation (r = 0.78, SEE=0.124, p < 0.0001) between calculated and postmortem LV mass (Litwin et al. 1994, 1995).
 |
where IVSd is the left ventricular septal thickness diastole, LVIDd is the left ventricular internal dimension during diastole, and PWTd is the posterior wall thickness diastole (Aigbe et al. 2012).
Serum collection
During tissue collection blood was collected by cardiac puncture into a BD Vacutainer serum collection tube. The blood was centrifuged at 800 × g for 15 min to separate serum. The serum was collected and used to measure serum parameters using an Abaxis VetScan analyzer (Abaxis, Union City, CA, USA). The following parameters were determined non-fasting serum glucose (GLU), alanine aminotransferase (ALT), alkaline phosphatase (ALP), blood urea nitrogen (BUN), albumin (ALB), calcium (Ca2+), creatinine (CRE), amylase (AMY), globulin (GLOB), potassium (K+), sodium (Na+), phosphorus (PHOS), total bilirubin (TBIL), and total protein (Drexler et al. 2003).
Heart collection
Rats were anesthetized with an intraperitoneal injection of ketamine (40 mg/kg) and xylazine (10 mg/kg) and supplemented as necessary for reflexive response. Before heart collection, a three lead electrocardiogram (EKG) was performed on all animals using the Biopac Student Lab software (BIOPAC Systems, Inc., Microsoft). After completion of the EKG, the heart was removed after a midline laparotomy and placed in Krebs–Ringer bicarbonate buffer (KRB) containing the following: 118 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 24.2 mM NaHCO3, and 10 mM α-d-glucose (pH 7.4) equilibrated with 5 % CO2/95 % O2 and maintained at 37 ° C. Isolated hearts were quickly massaged to remove any blood from the ventricles, cleaned of connective tissue, weighed, and immediately snap frozen in liquid nitrogen.
Electrocardiographic analysis
EKGs collected from the aging female rats were analyzed using BioPac Student Lab PRO software. All animals were evaluated for any electrical anomalies in all three leads. Lead II was used to evaluate changes in EKG parameters, six complete cardiac cycles from the Lead II tracing were used to obtain data for comparison of ventricular acceleration time (VAT), heart rate, ST interval, T amplitude, QRS interval, QT interval, PR interval, T duration, T–T interval, R + S amplitude, S amplitude, R amplitude, Q amplitude, and P amplitude (Fig. 3a). Mean electrical axis was calculated from leads I and III after calibration of 2 cm/mV (Tsika et al. 2010) using the following formula derived by Singh and Athar (Singh and Sajjad 2003).
 |
Fig. 3.
EKG in the aging female F344xBN heart. a EKG interval diagram and b representative EKG tracing of leads I, II, and III obtained from 6-, 26-, and 30-month-old females.
Heart rate was determined by the average intervals between R waves on lead II.
Determination of myocyte cross-sectional area (CSA) and histological analysis
An IEC Minotome Cryostat was used to section (8 μm) frozen hearts (n = 4) on poly-lysine coated slides. To determine morphology, heart sections were stained with hematoxylin and eosin stain. Picrosirius red staining was employed to examine collagen. The collagen area fraction of the picrosirius red stained tissues was determined using Image J software.
Immunostaining for dystrophin (NCL-DYS2, Novocastra Vector Laboratories, Burlingame, CA, USA) and connexin-43 (#3512, Cell Signing Technology, Danvers, MA, USA) was visualized by immunofluorescence as outlined by the antibody manufacturer. A phosphate-buffered saline (PBS) containing 0.5 % Tween-20 (PBS-T) at pH 7.5 was used to wash sections three times before incubating for 30 min in a blocking solution (5 % BSA). In a humidified chamber at 24 °C, the dystrophin antisera diluted in PSB-T (antibody dilution of 1:100) was added to sections for 1 h. After incubation the sections were washed three time with PBS, and incubated again for 30 min in a humidified chamber at 24 °C with a secondary antibody solution containing an FITC anti-rabbit IgG (1:200) and DAPI (1.5 μg/ml) in order to visualize cell nuclei. Before mounting sections were washed a final time with PBS. The epifluorescence of specimens was visualized using an Olympus fluorescence microscope (Olympus, Melville, NY, USA) fitted with a 40x objective. Images were recorded digitally using A CCD camera and the Olympus MicroSuite™ Basic from Olympus America (Olympus) were used to digitally record and analyze images, respectively. The CSA of the cardiac myocytes was determined by measuring the area within the dystrophin positive staining.
Statistics
Results are given as mean ± SEM. Statistical analyses were performed using Sigma Stat 3.5 statistical software (Systat Software, Inc.). Age comparisons between echocardiographic structural, functional parameters, and morphologic indices were evaluated by one-way ANOVA with the Student–Newman–Keuls post hoc test for parametric normally distributed data or Kruskal–Wallis one-way analysis of variance on Ranks with a Dunn's post hoc test for none parametric distributions. Linear regression analysis was performed with dependent variables against the independent variables age, correlation were ranked as low correlation (0.3 to 0.7), moderate correlation (0.5 to 0.7), high correlation (0.7 to 0.9), very high correlation (0.9 to 1) between parameters (supplemental data). The level of significance accepted a priori was ≤0.05.
Results
Cardiac function is preserved in the aging female F344xBN rat
Compared to 6-month-old animals, heart and body weight was higher at 26 months (72 ± 2 vs. 95 ± 2 mg, p < 0.001; 235.3 ± 1.7 vs. 296.4 ± 6.5 g; p < 0.05) and 30 months (104 ± 4 mg, p < 0.05); 235.3 ± 1.7 vs. 315.6 ± 8.6 g; p < 0.05) (Table 1). Compared to 6-month-old animals, cardiac myocyte CSA was 20 ± 1.9 % and 28 ± 2.3 % higher in the 26- and 30-month-old animals, respectively (p < 0.05) (Fig. 1). Histological analysis using picrosirius red staining demonstrated a significant age-related decrease in collagen reactivity (Fig. 1) (p < 0.05). Immunohistochemical staining of the aging female heart suggested alterations in Cx43 localization from the cell ends to the lateral margins (Fig. 2a). Alignment of the Cx43 staining appears to be uniform, localized, and linear within the 6-month tissues sections, as would be expected because of Cx43 localization along the intercalated disk. The linearity of the staining appears to become less uniform and diffuse with aging which may indicate alteration in Cx43 distribution within the cardiacmyocyte. In addition to changes in Cx43 immunoreactivity, aging also appeared to be associated with changes in cardiac rhythm. In particular, aging appeared to be characterized by the presence of hyper-acute T waves, a long PR interval, a long P wave duration, tall P waves, and significant Q waves (Fig. 3b, Table 2). No evidence of arrhythmias was observed in any age group.
Table 1.
Blood parameters for the aging female F344xBN rat (mean ± SEM)
| Parameters | 6 months | 26 months | 30 months |
|---|---|---|---|
| ALB (g/dl) | 4.4 ± 0.03 | 4.4 ± 0.1 | 4.2 ± 0.1 |
| ALP (μ/l) | 217.9 ± 10.7 | 168.0 ± 9.6* | 167.8 ± 7.4* |
| ALT (μ/l) | 45.1 ± 2.2 | 57.6 ± 3.7 | 49.8 ± 2.4 |
| AMY (μ/l) | 551.1 ± 22.1 | 640.3 ± 29.6* | 589.8 ± 18.0 |
| TBIL (mg/dl) | 0.2 ± 0.02 | 0.2 ± 0.02 | 0.3 ± 0.03 |
| BUN (mg/dl) | 17.5 ± 0.4 | 15.4 ± 0.6* | 15.7 ± 0.5* |
| Ca2+ (mg/dl) | 10.7 ± 0.09 | 11.2 ± 00.13* | 11.0 ± 0.1 |
| PHOS (mg/dl) | 10.2 ± 0.4 | 8.1 ± 0.2* | 7.8 ± 0.2* |
| CRE (mg/dl) | 0.3 ± 0.02 | 0.4 ± 0.02 | 0.4 ± 0.03* |
| GLU (mg/dl) | 372.8 ± 16.0 | 346.9 ± 10.3 | 323.7 ± 13.3* |
| Na+ (mmol/l) | 141.8 ± 0.9 | 142.0 ± 0.6 | 141.1 ± 0.5 |
| K+ (mmol/l) | 7.12 ± 0.06 | 6.4 ± 0.14* | 6.0 ± 0.11* |
| TP (g/dl) | 5.8 ± 0.05 | 6.4 ± 0.08* | 6.3 ± 0.11* |
| GLOB (g/dl) | 1.56 ± 0.12 | 2.00 ± 0.10* | 2.12 ± 0.11* |
| Ca2+/PHOS (ratio) | 1.03 ± 0.12 | 1.39 ± 0.12* | 1.44 ± 0.14* |
| Na+/K+ (ratio) | 19.90 ± 0.66 | 22.40 ± 1.99* | 23.58 ± 1.94* |
| BUN/CRE (ratio) | 68.96 ± 16.1 | 46.77 ± 16.9* | 49.64 ± 17.6* |
| ALB/GLOB (ratio) | 3.29 ± 0.68 | 3.32 ± 0.61* | 2.14 ± 0.60* |
Six-month-old animals; 26-month-old animals; 30-month-old animals
*p < 0.05, significantly different from 6-month-old animals; n = 4–6 per group
Fig. 1.
CSA, tissue morphology, and fibrosis in the aging female F344xBN heart. a Immunohistochemical staining of cardiac tissue stained with Dystrophin. Bar = 100 μm. b Cross-sectional area of cardiac myocytes as determined by dystrophin staining. c Hematoxylin and Eosin and d Picrosirius Red staining of 6-, 26-, and 30-month-old female F344xBN hearts. (*p < 0.05) significantly different from 6-month-old animals
Fig. 2.
Age-associated changes in connexin 43 distribution in the aging female F344xBN heart. Distribution of connexin 43 in a 6-, b 26-, and c 30-month-old female F344xBN hearts. Bar = 50 μm
Table 2.
Total body weight (BW) and heart weight (HW) in female F344xBN rats at 6, 26, and 30 months of age (means ± SEM)
| Groups | 6 months | 26 months | 30 months |
|---|---|---|---|
| n | 4 | 22 | 10 |
| Heart weight (g) | 0.72 ± 0.02 | 0.95 ± 0.02* | 1.0 ± 0.04*† |
| Body weight (g) | 235.3 ± 1.7 | 296.4 ± 6.5* | 315.6 ± 8.6* |
| HW/BW ratio (%) | 0.317 ± 0.02 | 0.32 ± 0.01 | 0.33 ± 0.01 |
Six-month-old animals; 26-month-old animals; 30-month-old animals
*p < 0.05, significantly different from 6-month-old animals
† p < 0.05, significantly different from 26-month-old animals
No age-associated changes in E–E′ ratio were found in the female F344xBN rats. However, E′ was significantly reduced in the 30-month age group when compared to 6- and 26-month age groups (Fig. 4; p < 0.05). Left ventricular isovolumic relaxation time was significantly higher in 26-month-old animals (0.036 ± 0.005 s) compared to that seen in 6-month-old animals (0.015 ± 0.002 s; p < 0.05). Mitral valve deceleration time demonstrated no significant change with age. Mitral valve Emax significantly lower at 26 (61.5 ± 1.5 cm/s) and 30 months (55.4 ± 1.4 cm/s) when compared to that seen in the 6-month-old animals (78.0 ± 3.3 cm/s; p < 0.05). Mitral valve Amax levels were lower at 30 months (35.1 ± 1.6 cm/s) when compared to 6 months (41.5 ± 1.3 cm/s; p < 0.05). No significant changes were found in MV E/A ratio with age (Table 3).
Fig. 4.
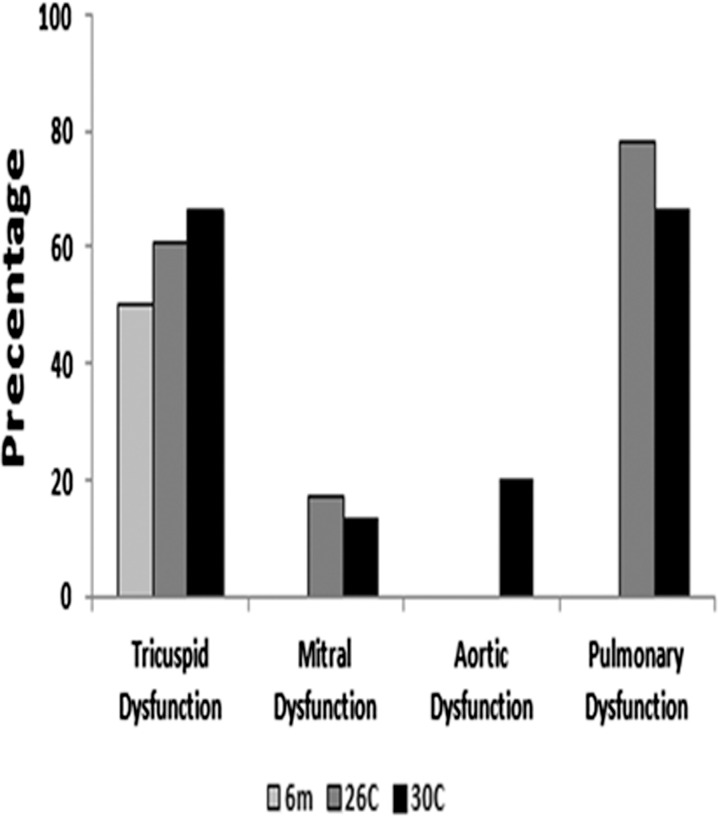
Age-associated valve dysfunction in the female F344xBN heart. Percentage of female F344xBN rats with valve dysfunction at 6, 26, and 30 months
Table 3.
Echocardiographic evaluation of cardiac functional parameters in aging female F344xBN rats (mean ± SEM)
| Groups | 6 months | 26 months | 30 months |
|---|---|---|---|
| E–E′ | 20.8 ± 1.02 | 14.7 ± 0.64 | 21.9 ± 2.63 |
| E′ | 3.89 ± 0.14 | 3.42 ± 0.42 | 2.71 ± 0.19* |
| LV IVRT (s) | 0.015 ± 0.002 | 0.036 ± 0.005* | 0.030 ± 0.000 |
| MV Dec Time (s) | 0.053 ± 0.005 | 0.057 ± 0.002 | 0.063 ± 0.003 |
| MV Emax (cm/s) | 78 ± 3.3 | 62 ± 1.5* | 55 ± 1.4* |
| MV Amax (cm/s) | 42 ± 1.3 | 36 ± 0.9 | 35 ± 1.6* |
| MV E/A | 1.75 ± 0.03 | 1.60 ± 0.06 | 1.66 ± 0.10 |
| Ejection fraction (%) | 74 ± 0.9 | 82 ± 1.0* | 78 ± 1.8 |
| Fractional shortening (%) | 38 ± 0.7 | 46 ± 1.0 | 42 ± 1.7 |
| ESV (ml) | 0.143 ± 0.017 | 0.082 ± 0.005* | 0.114 ± 0.10 |
| EDV (ml) | 0.535 ± 0.052 | 0.492 ± 0.20 | 0.523 ± 0.022 |
| Heart rate (bpm) | 281 ± 16.5 | 259 ± 8.8 | 278 ± 10.1 |
Six-month-old animals; 26-month-old animals; 30-month-old animals
*p < 0.05, significantly different from 6-month-old animals
Compared to 6-month-old animals, ejection fraction was significantly higher at 26 months (82 ± 1.0 % vs. 74 ± 0.9 %; p < 0.05). Fractional shortening was lower at 30 months (41.5 ± 1.7 %) when compared to that observed in 26-month-old animals (45.6 ± 1.0 %; p < 0.05). End systolic volume was significantly lower at 26 months (0.082 ± 0.005 ml) when compared to 6-month-old animals (0.143 ± 0.017 ml; p < 0.05). However, heart rate was unaltered with age (Table 4).
Table 4.
Electrocardiographic evaluation of cardiac conduction parameters in aging female F344xBN rats (mean ± SEM)
| EKG Measurements | 6 months | 26 months | 30 months |
|---|---|---|---|
| VAT | 0.036 ± 0.0013 | 0.044 ± 0.0010* | 0.046 ± 0.0007* |
| ST interval | 0.055 ± 0.0035 | 0.080 ± 0.0018* | 0.073 ± 0.0013* |
| T amplitude | 0.090 ± 0.0065 | 0.123 ± 0.0071* | 0.098 ± 0.0080† |
| QRS interval | 0.055 ± 0.0010 | 0.066 ± 0.0013* | 0.070 ± 0.0005*† |
| QT interval | 0.110 ± 0.0035 | 0.146 ± 0.0023* | 0.143 ± 0.0012* |
| PR interval | 0.035 ± 0.0027 | 0.033 ± 0.0015 | 0.032 ± 0.0010 |
| T duration | 0.055 ± 0.0035 | 0.080 ± 0.0018* | 0.073 ± 0.0013* |
| T–T interval | 0.217 ± 0.0034 | 0.217 ± 0.0032 | 0.218 ± 0.0050 |
| R + S amplitude | 0.321 ± 0.0105 | 0.280 ± 0.0161 | 0.276 ± 0.0247 |
| S amplitude | −0.009 ± 0.0061 | −0.007 ± 0.0049 | −0.029 ± 0.0121 |
| R amplitude | 0.035 ± 0.0027 | 0.033 ± 0.0015 | 0.032 ± 0.0010 |
| Q amplitude | −0.005 ± 0.0019 | −0.014 ± 0.0024* | −0.018 ± 0.0017* |
| P amplitude | 0.006 ± 0.0052 | 0.037 ± 0.0018* | 0.037 ± 0.0020* |
| P duration | 0.035 ± 0.0027 | 0.033 ± 0.0015 | 0.032 ± 0.0010 |
| Mean electrical axis | 34.41 ± 5.99 | 63.13 ± 4.86* | 63.26 ± 3.72* |
KG amplitudes measured on Lead II axis of 6-month-old animals; 26-month-old animals; 30-month-old animals
*p < 0.05, significantly different from 6-month-old animals
† p < 0.05, significantly different from 26-month-old animals
Aging increases septal and posterior wall thickness and valvular insufficiency in the female F344xBN heart
Compared to 6-month-old animals, left ventricular septal thickness (IVS) during systole was higher in 26-month-old animals (0.193 ± 0.008 vs. 0.253 ± 0.003 cm; p < 0.05). Left ventricular internal dimension during systole (LVIDs) significantly increased at 26 months (0.331 ± 0.009 cm) compared to 6 months (0.378 ± 0.017 cm; p < 0.05). During diastole LVID demonstrated no significant change. Left ventricular posterior wall thickness (LVPW) during systole and diastole was significantly increased at 30 months (LVPWs: 0.288 ± 0.009 cm; LVPWd: 0.189 ± 0.008 cm) compared to 6 months (LVPWs: 0.193 ± 0.002 cm; LVPWd: 0.153 ± 0.010 cm; p < 0.05). No changes were found in right ventricular dimension during diastole or left ventricular mass with age (Table 5).
Table 5.
Echocardiographic evaluation of cardiac structural parameters in aging female F344xBN rats (mean ± SEM)
| Groups | 6 months | 26 months | 30 months |
|---|---|---|---|
| IVSs (cm) | 0.193 ± 0.008 | 0.253 ± 0.003* | 0.239 ± 0.013 |
| IVSd (cm) | 0.118 ± 0.012 | 0.151 ± 0.003 | 0.149 ± 0.004 |
| LVIDs (cm) | 0.378 ± 0.017 | 0.331 ± 0.009* | 0.356 ± 0.011 |
| LVIDd (cm) | 0.610 ± 0.022 | 0.593 ± 0.009 | 0.606 ± 0.10 |
| LVPWs (cm) | 0.193 ± 0.002 | 0.267 ± 0.007 | 0.288 ± 0.009* |
| LVPWd (cm) | 0.153 ± 0.010 | 0.166 ± 0.004 | 0.189 ± 0.008* |
| RVDd (cm) | 0.105 ± 0.016 | 0.138 ± 0.009 | 0.111 ± 0.006 |
| LVM (g) | 0.487 ± 0.10 | 0.658 ± 0.09 | 0.646 ± 0.04 |
Six-month-old animals; 26-month-old animals; 30-month-old animals
*p < 0.05, significantly different from 6-month-old animals
The incidence of tricuspid, mitral, and pulmonary valve insufficiency was higher in 26- and 30-month-old female hearts relative to that observed in 6-month-old animals. The presence of aortic insufficiency with age was only seen in the 30-month age group (Fig. 4).
Aging is associated with alterations in serum glucose and electrolytes in the female F344xBN rat
In the aging female F344xBN non-fasting serum glucose levels were significantly decreased in 30 month-old (323.7 ± 13.3) compared to 6-month-old (372.8 ± 16.0) female F344xBN rats. Serum levels of ALP, BUN, PHOS, and potassium significantly decreased at 26 and 30 months compared to that observed in the 6-month-old animals. TP, GLOB, and CRE increased significantly at 30 months (6.3 ± 0.1, 2.12 ± 0.1, and 0.4 ± 0.03, respectively) compared to 6 months (5.8 ± 0.05, 1.56 ± 0.12, and 0.3 ± 0.002, respectively). In the 26-month-old female F344xBN rat serum calcium (11.2 ± 0.1) levels were significantly increased compared to 6-month-old female rats (10.7 ± 0.09). No significant changes were found in serum ALB, ALT, sodium, and TBIL with age in the female F344xBN. Significant evaluation of calcium to phosphate (Ca+/PHOS) and sodium to potassium (Na+/K+) were observed in the 26- and 30-month age groups when compared to 6-month-old animals (p < 0.05). Compared to 6-month-old animals, BUN/CRE and the albumin to globulin ration (ALB/GLOB) were lower in the 26- and 30-month age groups (Table 1) (p < 0.05).
Discussion
The purpose of this study was to examine how aging process affects the cardiac structure and function in the female F344xBN rat. Similar to previous work using male animals (Walker et al. 2006), our data indicated that aging in these animals is associated with an increase in the thickness of the septal and posterior walls between 6 and 30 months of age. In addition to these changes, we also observed that aging was associated with increases in average cardiomyocyte muscle fiber CSA (Fig. 1) and a trend towards increased left ventricular wall thickness (Table 5). These data, taken together, are consistent with the efforts of Boluyt and colleagues (Boluyt et al. 2004), who noted an increase in thickness of septal and posterior walls at 30 months in the aging F344 model. Unlike this study, previous echocardiography studies in humans, male F344xBN rats, and female F344 rats (at 30 months of age) have shown that left ventricular mass in female 344 rats is increased with age (Hacker et al. 2006; Walker et al. 2006; Olivetti et al. 1995; Hayward et al. 2000). Why discrepancies may exist between studies is currently unclear but may be related to differences in experimental design, differences across species, rat strain or in the time points chosen for examination. Future experiments perhaps using older female F344xBN rats may be useful for clarification.
Aging is oftentimes associated with an increased risk of diastolic dysfunction which appears to exhibit a greater incidence in women compared to men (Hamlin et al. 2004). Diastolic problems oftentimes, but not always, precede the development of systolic dysfunction (Hamlin et al. 2004). In humans, diastolic dysfunction is defined by abnormal ventricular relaxation and filling that is characterized by a low E wave velocity, a high A wave velocity, prolonged deceleration time, and prolonged isovolumetric relaxation (Hamlin et al. 2004). In the present study, we observed age-related decrease in E′, increases in left ventricular relaxation time and a trend in mitral valve deceleration time in the female F344xBN heart. Nonetheless, we did not find any changes in the E/A ratio with age. Conversely, Boluyt and colleagues (Boluyt et al. 2004), using aged female F344 animals demonstrated increases in LV IVRT, decreases in the E wave, and an increased A wave velocity. Similar to previous findings by Boluyt and colleagues (Boluyt et al. 2004), who used the aging female F344 model, we observed age-related increases in left ventricular relaxation time, decreased E′ wave velocity, and a trend in mitral valve deceleration time but no change in the E/A ratio with aging in the female F344xBN heart. Taken together, these data suggest that aging in the female F344 and F344xBN rats, like that seen in humans, is often characterized by alterations in diastolic function.
Like prior work done in F344 rat strain, aging in the female F344xBN rat did not appear to significantly impair systolic function (Boluyt et al. 2004; Forman et al. 1997). Similar to that observed in the aging F344 animals, we also noted a slight increase in ejection fraction at 26 months, although this parameter must be interpreted with caution since animals at this time point also exhibited increased evidence of valvular regurgitation (Boluyt et al. 2004). Interestingly, work by Forman and colleagues reported that F344 males had a higher occurrence of mitral regurgitation (MR) than that seen in their female counterparts which may help explain the differences in function between the sexes (Forman et al. 1997). This finding is consistent with previous work from our laboratory male F344xBN which demonstrating a higher percentage of MR with age (unpublished data). Why differences may exist between aged male and female F344xBN rats will require further investigation.
Probabilities of survival curves generated by the NIA were used in the current study in an effort to approximate aging humans in the 3rd, 7th, and 8th decades of life. It is likely that aging in the female F344xBN does not closely mimic the changes seen in aging women at similar time points along the aging spectrum. Why aging in female rodents might differ from that seen in humans is likely complex and poorly understood but may be related to dissimilarities in hormonal regulation during aging. Previous data has demonstrated that the F344XBN estrous cycle ceases at 16 months of age (Sone et al. 2007) which suggests that the 26- and 30-month-old animals used in the present study are likely to represent animals that are moderate and late post-menopausal. Unlike that seen in humans, this loss in function is characterized as persistent estrous with maintained levels of estrogen, lower levels of progesterone, the absence of luteinizing hormone surges, and ovulation. This initial decline, in turn, is followed by the final stage where aged female rodents have low levels of plasma estradiol, progesterone, and (no or little) developing ovarian follicles (Wu et al. 2005). Whether estrogen, if present, may have blunted the age-associated changes typically seen in male animals is currently unclear.
Age-associated alterations in heart rhythm the female F344xBN heart
Unlike the aging male F344xBN rats no evidence of arrhythmias were detected with age in the female F344xBN (Walker et al. 2006). Nonetheless, our data suggest that aging in the female F344xBN is associated with changes in heart rhythm including increases in the VAT, ST interval, T amplitude, QRS interval, QT interval, T duration, Q amplitude, P amplitude, and a shift in the mean electrical axis with age (Table 4), increase in heart weight, myocyte CSA, and LVM. Alterations in heart rhythm intervals such as increased PR interval, P wave amplitude, and QRS complex are oftentimes indicative of myocardial disease (Bestetti and Oliveira 1990). Previous studies have shown that aged male rats (20 months) had no difference in R–R interval, P wave, as well as QRS length; however, PR and QT interval were increased with age (da Silva 2002). Our current findings support the existence of alterations in cardiac conductance but provide little information as to the underlying mechanism. The elevation in VAT and QRS interval suggest that aging might not only affect depolarization but that it may also be associated with changes in the ability of the heart to undergo repolarization. Correlation analysis demonstrated a high correlation between heart weight to MV Amax velocity (0.752) and MV Amax velocity to CSA (0.827) (Tables S1–S4). Because the nature of correlation does not denote causation, it is important to note that further research is required to delineate the nature of these correlations and to better elucidate the causation of the age-associated changes listed in this study. Similarly, whether the changes in VAT and QRS interval we observed in the current study are due to differences in ion handling, ion channel density, alterations how electrical signals may propagate through the myocardium or if they reflect age-associated increases in myocyte CSA or chamber dimensions (Fig. 1, Table 3) is currently unclear and will require additional experimentation.
Heat rhythm propagation is dependent on cell-to-cell coupling between cardiomyocytes and that this coupling is dependent, at least in part, by ion channels and gap junctions. Previous studies have shown that age-related changes in impulse propagation may be related to abnormalities in the pattern of ventricular activation (Rossi et al. 2008). Recent data has suggested that alterations in heart rhythm intervals during aging may be associated with alterations in Cx43 expression/activation/heterogeneity, fibrosis, and hypertrophy (Rossi et al. 2008; Hayashi et al. 2002; Xiao et al. 2007; Howarth et al. 2008; Jansen et al. 2012). Other work examining Cx43 has demonstrated increases in atrial fibrillation in patients exhibiting increased heterogeneity of Cx43 distribution (Nattel et al. 2007). These possibilities appear to be consistent with our data, where we observed to be qualitative alterations in Cx43 localization with aging in the female F344xBN heart (Fig. 2). Future experiments to directly investigate if or how changes in Cx43 protein abundance and localization may affect cardiac rhythm in the aging female F344xBN rat model may be helpful in delineating whether Cx43 may play a role in the aging female heart.
In addition, we also examined if age-related changes in heart rhythm were associated with alterations in blood chemistry. It has been suggested that electrolyte disorders can affect the ion currents in the heart and that such changes might be related to the development of cardiac arrhythmias (El-Sherif and Turitto 2011). For example, previous studies have shown that decreased plasma sodium, potassium, and ionized calcium levels are associated with a higher risk of arrhythmias in hemodialysis patients (Berta et al. 2012; Tong and Hou 2006). Although very little is known about the role of serum electrolytes in influencing cardiac function during aging, alterations in serum electrolytes have been associated with increased all-cause mortality among patients suffering from coronary heart disease (Grandi et al. 2012). For the most part, we found that age-related changes in blood chemistry appeared to be within the normal range. Recent work has suggested that decreases in plasma potassium may be related to the development of tachyarrhythmias (Sabir et al. 2008). Whether the age-associated reduction in potassium levels seen in this study, although within the normal physiological range, might play a role in the changes seen within the EKG data and will require further investigation.
In conclusion, this study provides reference values for cardiac structure and function in the aging female F344xBN heart. Taken together, our data suggest that the female, unlike the male, F344xBN rat demonstrates subtle age-associated changes in cardiac structure, function, and conductance. The age-associated increase in heart wall thickness in the absence of fibrosis and increase heterogeneity of Cx43 distribution may partially explain the age-associated alterations in heart rhythm intervals. The possibility of altered serum ion levels may also contribute to reduced conductance. Further study is needed to better understand the mechanistic basis of how aging may affect cardiac structure and function in the female F344xBN rat.
Electronic supplementary material
(DOCX 72 kb)
(DOCX 71 kb)
(DOCX 72 kb)
(DOCX 75 kb)
(DOCX 73 kb)
Abbreviations
- F344xBN
Fischer 344/NNiaHSd × Brown Norway/BiNia F1 hybrid
- Cx43
Connexin 43
- AAALAC
Association for Assessment and Accreditation of Laboratory Animal Care International
- GLU
Glucose
- ALT
Alanine aminotransferase
- ALP
Alkaline phosphatase
- BUN
Blood urea nitrogen
- ALB
Albumin
- Ca2+
Calcium
- CRE
Creatinine
- AMY
Amylase
- GLOB
Globulin
- K+
Potassium
- Na+
Sodium
- PHOS
Phosphorus
- TBIL
Total bilirubin
- EKG
Electrocardiogram
- KRB
Krebs–Ringer bicarbonate buffer
- VAT
Ventricular acceleration time
- PBS
Phosphate-buffered saline
- PBS-T
PBS containing 0.5 % Tween-20
- BSA
Bovine serum albumin
- CSA
Cross-sectional area
- LVM
Left ventricular mass
- LVIDd
Left ventricular internal dimension during diastole
- LVIDs
Left ventricular internal dimension during systole
- IVSs
Left ventricular septal thickness systole
- IVSd
Left ventricular septal thickness diastole
- LVPWd
Left ventricular posterior wall thickness diastole
- LVPWs
Left ventricular posterior wall thickness systole
- RVDd
Right ventricular end-diastolic dimensions
- PWTd
Posterior wall thickness diastole
- FS
Fractional shortening
- EF
Ejection fraction
- TV
Tricuspid valve
- AV
Aortic valve
- PV
Pulmonary valve
- BW
Body weight
- HW
Heart weight
- MR
Mitral regurgitation
- NIA
National Institutes of Aging
- ESV
End systolic volume
- EDV
End diastolic volume
- LV IVRT
Left ventricular isovolumic relaxation time
Footnotes
J. Fannin and K.M. Rice contributed equally to this study.
References
- Aigbe IF, Kolo PM, Omotoso AB. Left ventricular structure and function in black normotensive type 2 diabetes mellitus patients. Ann Afr Med. 2012;11(2):84–90. doi: 10.4103/1596-3519.93530. [DOI] [PubMed] [Google Scholar]
- Anversa P, et al. Myocyte cell loss and myocyte cellular hyperplasia in the hypertrophied aging rat heart. Circ Res. 1990;67(4):871–885. doi: 10.1161/01.RES.67.4.871. [DOI] [PubMed] [Google Scholar]
- Berta E, et al. Evaluation of the metabolic changes during hemodialysis by signal averaged ECG. Pharmazie. 2012;67(5):380–383. [PubMed] [Google Scholar]
- Bestetti RB, Oliveira JS. The surface electrocardiogram: a simple and reliable method for detecting overt and latent heart disease in rats. Braz J Med Biol Res. 1990;23(12):1213–1222. [PubMed] [Google Scholar]
- Betsuyaku T, et al. Cardiac structure and function in young and senescent mice heterozygous for a connexin43 null mutation. J Mol Cell Cardiol. 2002;34(2):175–184. doi: 10.1006/jmcc.2001.1499. [DOI] [PubMed] [Google Scholar]
- Boluyt MO, et al. Echocardiographic assessment of age-associated changes in systolic and diastolic function of the female F344 rat heart. J Appl Physiol. 2004;96(2):822–828. doi: 10.1152/japplphysiol.01026.2003. [DOI] [PubMed] [Google Scholar]
- da Silva D. V.J., et al., Chronic converting enzyme inhibition normalizes QT interval in aging rats. Braz J Med Biol Res. 2002;35(9):1025–1031. doi: 10.1590/S0100-879X2002000900003. [DOI] [PubMed] [Google Scholar]
- Drexler E, et al. An experimental method for measuring mechanical properties of rat pulmonary arteries verified with latex. J Res Natl Stand Technol. 2003;108:183–191. doi: 10.6028/jres.108.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sherif N, Turitto G. Electrolyte disorders and arrhythmogenesis. Cardiol J. 2011;18(3):233–245. [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Forman DE, et al. Cardiac morphology and function in senescent rats: gender-related differences. J Am Coll Cardiol. 1997;30(7):1872–1877. doi: 10.1016/S0735-1097(97)00411-7. [DOI] [PubMed] [Google Scholar]
- Grandi NC, et al. Calcium, phosphate and the risk of cardiovascular events and all-cause mortality in a population with stable coronary heart disease. Heart. 2012;98(12):926–933. doi: 10.1136/heartjnl-2011-300806. [DOI] [PubMed] [Google Scholar]
- Hacker TA, et al. Age-related changes in cardiac structure and function in Fischer 344 × Brown Norway hybrid rats. Am J Physiol Heart Circ Physiol. 2006;290(1):H304–H311. doi: 10.1152/ajpheart.00290.2005. [DOI] [PubMed] [Google Scholar]
- Hamlin SK, et al. Role of diastole in left ventricular function: II. Diagnosis and treatment. Am J Crit Care. 2004;13(6):453–466. [PubMed] [Google Scholar]
- Hayashi H, et al. Aging-related increase to inducible atrial fibrillation in the rat model. J Cardiovasc Electrophysiol. 2002;13(8):801–808. doi: 10.1046/j.1540-8167.2002.00801.x. [DOI] [PubMed] [Google Scholar]
- Hayward CS, Kelly RP, Collins P. The roles of gender, the menopause and hormone replacement on cardiovascular function. Cardiovasc Res. 2000;46(1):28–49. doi: 10.1016/S0008-6363(00)00005-5. [DOI] [PubMed] [Google Scholar]
- Howarth FC, et al. Effects of streptozotocin-induced diabetes on connexin43 mRNA and protein expression in ventricular muscle. Mol Cell Biochem. 2008;319(1–2):105–114. doi: 10.1007/s11010-008-9883-5. [DOI] [PubMed] [Google Scholar]
- Jansen JA, et al. Reduced heterogeneous expression of Cx43 results in decreased Nav1.5 expression and reduced sodium current that accounts for arrhythmia vulnerability in conditional Cx43 knockout mice. Heart Rhythm. 2012;9(4):600–607. doi: 10.1016/j.hrthm.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawara T, et al. Activation delay after premature stimulation in chronically diseased human myocardium relates to the architecture of interstitial fibrosis. Circulation. 2001;104(25):3069–3075. doi: 10.1161/hc5001.100833. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. Cardiovascular aging: perspectives From humans to rodents. Am J Geriatr Cardiol. 1998;7(6):32–45. [PubMed] [Google Scholar]
- Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III. Cellular and molecular clues to heart and arterial aging. Circulation. 2003;107(3):490–497. doi: 10.1161/01.CIR.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- Lancaster TS, Jefferson SJ, Korzick DH. Local delivery of a PKCepsilon-activating peptide limits ischemia reperfusion injury in the aged female rat heart. Am J Physiol Regul Integr Comp Physiol. 2011;301(5):R1242–R1249. doi: 10.1152/ajpregu.00851.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman RD, et al. Pathologic characterization of brown Norway, brown Norway × Fischer 344, and Fischer 344 × brown Norway rats with relation to age. J Gerontol A Biol Sci Med Sci. 1996;51(1):B54–B59. doi: 10.1093/gerona/51A.1.B54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin SE, et al. Serial echocardiographic assessment of left ventricular geometry and function after large myocardial infarction in the rat. Circulation. 1994;89(1):345–354. doi: 10.1161/01.CIR.89.1.345. [DOI] [PubMed] [Google Scholar]
- Litwin SE, et al. Serial echocardiographic-Doppler assessment of left ventricular geometry and function in rats with pressure-overload hypertrophy. Chronic angiotensin-converting enzyme inhibition attenuates the transition to heart failure. Circulation. 1995;91(10):2642–2654. doi: 10.1161/01.CIR.91.10.2642. [DOI] [PubMed] [Google Scholar]
- Nattel S, et al. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87(2):425–456. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- Olivetti G, et al. Gender differences and aging: effects on the human heart. J Am Coll Cardiol. 1995;26(4):1068–1079. doi: 10.1016/0735-1097(95)00282-8. [DOI] [PubMed] [Google Scholar]
- Rossi S, et al. Ventricular activation is impaired in aged rat hearts. Am J Physiol Heart Circ Physiol. 2008;295(6):H2336–H2347. doi: 10.1152/ajpheart.00517.2008. [DOI] [PubMed] [Google Scholar]
- Sabir IN, et al. Restitution analysis of alternans and its relationship to arrhythmogenicity in hypokalaemic Langendorff-perfused murine hearts. Pflugers Arch. 2008;455(4):653–666. doi: 10.1007/s00424-007-0327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PN, Sajjad M. Athar. Simplified calculation of mean QRS vector (mean electrical axis of heart) of electrocardiogram. Indian J Physiol Pharmacol. 2003;47(2):212–216. [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273(5271):59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone K, et al. Changes of estrous cycles with aging in female F344/n rats. Exp Anim. 2007;56(2):139–148. doi: 10.1538/expanim.56.139. [DOI] [PubMed] [Google Scholar]
- Tong Y, Hou H. The alteration of QT dispersion in hemodialysis subjects. Kidney Blood Press Res. 2006;29(4):231–236. doi: 10.1159/000095738. [DOI] [PubMed] [Google Scholar]
- Tsika RW, et al. TEAD-1 overexpression in the mouse heart promotes an age-dependent heart dysfunction. J Biol Chem. 2010;285(18):13721–13735. doi: 10.1074/jbc.M109.063057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turturro A, et al. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54(11):B492–B501. doi: 10.1093/gerona/54.11.B492. [DOI] [PubMed] [Google Scholar]
- Walker EM, Jr, et al. Age-associated changes in hearts of male Fischer 344/Brown Norway F1 rats. Ann Clin Lab Sci. 2006;36(4):427–438. [PubMed] [Google Scholar]
- Wu JM, et al. Ovarian aging and menopause: current theories, hypotheses, and research models. Exp Biol Med (Maywood) 2005;230(11):818–828. doi: 10.1177/153537020523001106. [DOI] [PubMed] [Google Scholar]
- Xiao J, et al. Resveratrol restored the structural and functional association between M3 receptor and connexin 43 gap junction proteins in ischemia–reperfusion injury of isolated rat heart. Yao Xue Xue Bao. 2007;42(1):19–25. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 72 kb)
(DOCX 71 kb)
(DOCX 72 kb)
(DOCX 75 kb)
(DOCX 73 kb)