Abstract
Epigenetic mechanisms play a fundamental role in generating diverse and heritable patterns of viral and cellular gene expression. Epstein-Barr Virus (EBV) can adopt a variety of gene expression programs that are necessary for long-term viral persistence and latency in multiple host-cell types and conditions. The latent viral genomes assemble into chromatin structures with different histone and DNA modifications patterns that control viral gene expression. Variations in nucleosome organization and chromatin conformations can also influence gene expression by coordinating physical interactions between different regulatory elements. The viral-encoded and host-cell factors that control these epigenetic features are beginning to be understood at the genome-wide level. These epigenetic regulators can also influence viral pathogenesis by expanding tissue tropism, evading immune detection, and driving host-cell carcinogenesis. Here, we review some of the recent findings and perspectives on how the EBV epigenome plays a central role in viral latency and viral-associated carcinogenesis.
Keywords: Epstein-Barr virus, gammaherpesvirus, chromatin, histone modifications, DNA methylation, chromosome conformation, CTCF, OriP
Introduction
Epstein-Barr virus (EBV) is a human gammaherpesvirus that can establish a life-long infection in 95% of the population worldwide [1]. The remarkable success of this viral pathogen can be partially attributed to its ability to establish a variety of gene expression programs that enable adaptation to different cell types and host-cell conditions. Variation in viral gene expression may also account for the broad range of viral-associated disease. EBV genomes and gene products are consistently detected in a diverse number of human cancers, including endemic Burkitt's lymphoma (BL), nasopharyngeal carcinoma (NPC), ∼50% of Hodgkin's disease, ∼10% of gastric carcinomas, and most lymphoproliferative disorders of immunosuppressed individuals [2, 3]. In each of these cancer-associated infections, EBV has a distinct gene expression program that reflects the host cell-type transcription factors, and ultimately, distinct epigenetic modifications of the viral genome. Epigenetic modifications are thought to generate diversity, as well as provide stability to gene expression programs in dividing cell populations. Here, we review how epigenetic modifications and chromatin organization play a central role in generating both diversity and stability of EBV gene expression programs during latent infection in various normal and cancer cell types.
EBV Latency Types
During latent infection, most EBV genomes persist as circular minichromosomes in the nucleus of infected cells (Fig. 1A) [1]. EBV gene expression during latency is highly restricted compared to the productive lytic cycle, and depends on the tissue or tumor type from which the EBV-positive cell-line was derived [4]. At least four different gene expression programs have been described, and are referred to as latency types [5]. In lymphoblastoid cell lines (LCLs) and B-cell lymphomas that occur during immunosuppression, EBV expresses the full set of latency associated genes. This least restrictive latency type is referred to as Type III latency, and consists of the expression of EBNA1, -2, -3A, -3B, -3C, -LP, the latency membrane proteins LMP1 and -2, and the non-coding RNAs (the EBERS, microRNAs, and the BARTs) [6]. All other latency types involve increasing degrees of viral gene silencing. For example, Type I latency consists of the expression of only one viral protein, EBNA1 and a few non-coding RNAs [7, 8]. The different latency types and their corresponding gene expression programs correlate with alternative utilization of transcription start sites and promoter elements [5, 9] (Fig. 1B). For example, in Type I latency, the EBNA1 gene is transcribed from the EBV Q promoter (Qp) localized in the BamHI Q region of the EBV genome [10], while in type III latency, a polycistronic mRNA coding for all the EBNA genes is initiated from the C promoter (Cp) in the BamHI W/C region of the EBV genome [5]. Also, promoter switching occurs during EBV immortalization and B-cell maturation, with transcription initiating at the W promoter (Wp) during primary infection and its subsequent switching to the upstream start sites controlled by Cp. The mechanisms that control promoter selection and switching during B-cell maturation are not completely understood. EBNA2 protein is required for strong activation of Cp, as well as LMP1, and its expression and function is closely coordinated with B-cell identity and proliferation factors [9, 11]. Some of the central players in B-cell development, like Pax5, Pu.1, and RBP-jK, are known to play similarly central roles in regulating EBV promoter function and latency type. Dynamic feed-forward and auto-repression mechanisms contribute to the establishment of a stable gene expression program for each latency type [12]. These programs are further reinforced by epigenetic changes on the viral genome.
Figure 1. Summary of EBV latency type gene expression and promoter utilization.
A. The EBV double-stranded genome map showing the position of regulatory elements and the location of latent transcripts, including non-coding RNA. The coordinates correspond to the 172 kbp reference viral genome. B. (i) Early stage transcription during primary infection of B-lymphocytes. Wp initiated transcription of EBNA2 is observed prior to Cp utilization. Qp may be used for generation of EBNA1 transcripts also required for the switch to Cp and establishment of Type III latency. (ii) Type III is observed in LCLs and in B-cell lymphomas associated with immunosuppression. Type III latency utilizes the Cp to generate several alternatively spliced and multicistronic transcripts that generate EBNA-LP, EBNA-2, EBNA-3A, EBNA-3B, EBNA-3C, and EBNA1. Type III latency also produces LMP1 and LMP2 transcripts from a convergent promoter region. Non-coding RNAs are also expressed in both latency types, but are not shown in this schematic. (iii) Type I latency is found in most Burkitt Lymphomas and is thought to occur during natural infection during B-cell maturation into memory B-cells. Type I latency utilizes Qp to generate EBNA1 only transcript.
Epigenetic Control of EBV Latency
Epigenetic programming plays a central role in regulating EBV gene expression and latency types [13]. Epigenetic programming involves DNA methylation, nucleosome positioning, histone tail modifications, and higher-ordered structures including promoter-enhancer loop formation. The importance of epigenetic modifications in the control of EBV latency has been known for over a decade as it has been well-established that pharmacological inhibitors of DNA methylation and histone deacetylation can both activate Type III promoters from Type I cells, as well as reactivate lytic gene transcription in some latently infected cell types [14, 15]. In latently infected cells, the majority of EBV genomes exist as non-integrated episomes with a nucleosomal pattern similar to that of host chromatin [16, 17]. Nucleosome position and density has been implicated in the regulation of most host DNA processes, and is also likely to regulate the transcription, replication, and repair of the latent EBV genomes. DNA methylation is typically associated with transcriptional repression, although there are many exceptions and EBV lytic activation is one of these exceptions [18]. Post-translation modifications of histone tails are among the best characterized epigenetic modifications, and can alter the chromatin structure in a reversible way that controls access to DNA [19]. Modifications of histones have complex and sometimes paradoxical roles in regulation of gene activity. In general, acetylation of Lysine 9 (H3K9ac) and methylation of Lysine 4 of histone H3 (H3K4me) mark regions of chromatin associated with active gene expression [19, 20]. In addition, the extent of lysine methylation can distinguish between different regulatory features: H3K4me1 marks enhancers; H3K4me2 marks both enhancers and promoters; H3K4me3 marks promoters and transcription starting sites (TSS) [21, 22]. Histone modifications associated with heterochromatin include methylation of lysine 9 (H3K9me3) [23, 24] and lysine 27 of histone H3 (H3K27me3) [25]: H3K9me3 marks regions associated with constitutive heterochromatin and repetitive elements; H3K27me3 marks regions repressed by polycomb complex, indicative of developmentally regulated genes [25]. Recent genome-wide studies have revealed that the EBV is subject to complex patterning of these histone modifications and that the patterns vary between latency types and correlate with different viral gene expression programs [26].
Histone Modifications on the EBV Genome
The chromatin composition of the EBV genome varies between different latency types and the histone modifications tend to correlate with expected marks at sites of transcriptional activation or repression. A high level of epigenetic variability between Type I and III latency occurs at the transcription start sites for the Type III-specific viral genes. The Cp and the LMP1/LMP2 promoters are enriched for acetylated histones (e.g. H3K9Ac, H3K27ac, H4 Ac) as well as for H3K4me3 in type III latency where they are actively transcribed. These euchromatic marks are absent from Cp and LMP1/LMP2 in Type I latency where these promoters are inactive [27]. In contrast, the Qp is associated with transcription activation marks, such as H3K9Ac, AcH4, H3K3m2 and H3K4me3 in Type I latency, where Qp is active, but not in Type III, where Qp is repressed [28, 29] (Fig. 2). Histone modifications associated with constitutive and facultative heterochromatin are also varied in different latency types. In Type I latency relatively high levels of H3K9me3 are observed at the W repeats, the Cp and the LMP1/2 promoters, which are transcriptionally repressed. In contrast, Type III infected cells show a general low level of H3K9me3 across the viral genome, as might be expected since all the latent genes are expressed [29, 30]. Interestingly H3K27me3 is weakly enriched at the lytic immediate early promoters in both Type I and Type III latency, suggesting that facultative heterochromatin associated with polycomb complex repression limits lytic reactivation [29, 31, 32]. H3K27me3 and polycomb-associated repression has been implicated in the control of KSHV latency, and may serve a similar function in EBV latency, as well [33, 34].
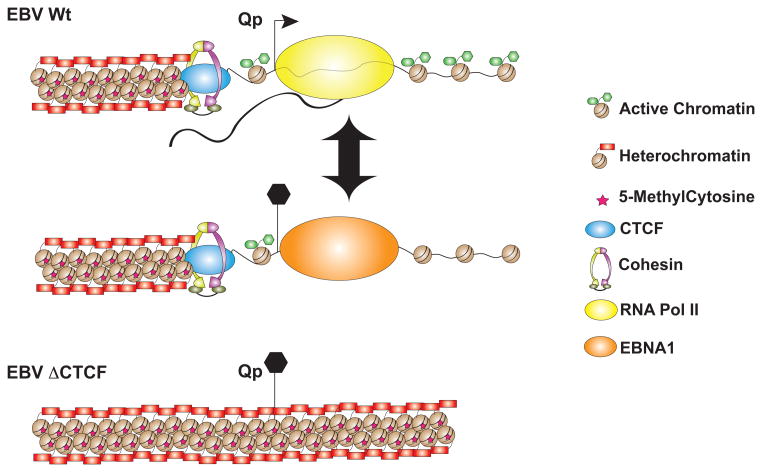
Figure 2. A Chromatin Boundary Function of CTCF at Qp.
CTCF binds upstream of the Qp TSS and is required for maintaining Qp activity in Type I latency. In Type III latency, Qp is repressed by EBNA1 binding to the TSS. In genomes where CTCF binding site is genetically disrupted at Qp, transcription initiation is gradually silenced by the invasion of H3K9me3 and DNA methylation. CTCF binding is also required for the activity of Qp in Type I and the stable binding of EBNA1 in Type III latency.
DNA Methylation of the EBV Genome
DNA methylation is known to play an important role in controlling cellular and viral gene expression. DNA methylation of promoter regions correlates with gene silencing and regulates several cellular processes including genomic imprinting, suppression of transposable elements, and X-chromosome inactivation [35-37]. In EBV DNA methylation levels has been explored both in Type I and III latently infected cell lines [38-40]. Following the infection of primary B-cells in vitro the EBV genome becomes progressively methylated at different regions including the W repeats, the C and the LMP1/2 promoters and sites for the initiation of transcription for lytic genes [28, 39, 41-44]. Naïve B-cells from healthy EBV positive donors as well as primary tissues from different EBV-positive lymphomas show similar DNA methylation pattern across the viral genome [45]. Throughout the EBV genome DNA methylation frequency overlaps with heterochromatic marks for H3K9me3, and correlates inversely with euchromatic histone modifications H3K9ac and H3K4me3. This is consistent with the reported interactions of DNMTs with histone H3K9me3, and underscores the cross-talk between epigenetic modification [46].
Several studies show that CpG methylation levels are elevated at the Cp and LMP1 promoters in Type I latency relative to Type III [28, 47, 48]. Treatment with the DNMT inhibitor 5-azacytidine can revert the restricted gene expression program in Type I latently infected cells, resulting in the reversal of methylation at the Cp and LMPs promoters, and the transcription reactivation of EBNAs, LMP1 and LMP2 mRNAs [15]. This clearly demonstrates the need for active DNA methylation to maintain stable repression of Cp and LMPs promoters during Type I latency. While DNA methylation can silence Cp and LMP promoters, DNA methylation is never observed at the Qp, despite the fact the Qp may be silenced in Type III latency [28, 43]. However, the Qp can be methylated when removed from its EBV genome context and inserted into a reporter expression constructs [49] indicating that Qp is protected from DNA methylation in the context of the viral genome.
How different methylation patterns are established between latency types it is not known. The viral protein LMP1 downregulates the expression of DNMT1 in germinal center B cells infected with EBV [50]; and DNMT1 and DNMT3B are upregulated in EBV positive cell lines supporting Type I latency compare to Type III latency [51]. However, the depletions of both DNMT1 and DNMT3B failed to reactivate lytic gene expression from the Type III latency or to induce Cp reactivation from Type I latency [51]. However, other studies have found that 5′ azacytidine can reactivate Cp transcription from some Type I cells [52]. These different findings may reflect different cell types and viral epigenotypes that respond differently to perturbations in epigenetic modifications.
DNA methylation also plays a central role in regulating EBV lytic cycle gene expression. While DNA methylation is typically associated with transcription repression, methylation of some viral promoter elements is essential for lytic cycle reactivation. This paradoxical effect is explained by the ability of the viral-encoded immediate early protein Zta (also referred to as ZEBRA, Z, and BZLF1) to selectively bind to methylated DNA at a subset of viral lytic promoters [53, 54]. EBV genomes are gradually methylated during the establishment of latency, but it is the lack of DNA methylation that prevents the completion of the viral lytic cycle. DNA methylation is required for Zta to bind promoter DNA of several essential viral genes [55, 56]. Zta has evolved the specialized capacity to overcome the silencing of lytic genes by selectively binding to methylated DNA [56, 57]. Interestingly, the Zta promoter is silenced by epigenetic mechanisms distinct form DNA methylation, including sequence specific repressor proteins like ZEB1 [58], and elevated H3K9me3 repressive marks that prevent lytic gene expression and replication during latency [30, 31]. Thus, DNA methylation plays both negative and positive roles in regulating EBV gene expression programs.
Chromatin insulators and three-dimensional structure of the EBV episome
Recent genome-wide ChIP-Seq studies of latently infected cells reveals that EBV genomes have complex and mottled epigenetic patterns [26]. In some cases, epigenetic modifications form a domain with a distinct boundary marked by the CCCTC-binding factor, CTCF [28]. CTCF is a highly conserved 11-zinc finger DNA binding protein that regulates different aspects of chromatin organization [59]. CTCF plays a crucial role in cellular processes such as gene expression, enhancer blocking, gene imprinting, and chromatin insulation [60, 61]. CTCF binding profile on EBV genome identified 19 CTCF binding sites, most of them at key regulatory regions including the Cp, the Qp and the LMP1/2 promoters [26, 28, 62]. The precise function of CTCF binding at a specific loci is not always obvious since CTCF can have multiple functions and operate at distal locations [60]. However some of the CTCF sites are situated perfectly to function as a border between epigenetic domains, suggesting that at these loci CTCF could function as a chromatin boundary factor [26, 28]. This is the case of the Qp promoter where CTCF binds upstream the transcription start site, bordering on a chromatin region enriched for H3K9me3 and DNA methylation (Fig. 2). When CTCF binding is removed by genetic manipulation of EBV genome the repressive chromatin marks can spread into the Qp start site and silence transcription [28]. The role of CTCF upstream of the Cp is less clear. Since CTCF binds between the Cp and OriP, which is known to work as a distal enhancer for this promoter, it has been proposed that CTCF could function as insulator and then negatively regulate Cp activity in Type I latently infected cells [63]. This model seems to be supported by the higher occupancy of CTCF at Cp in Type I compared to Type III latency [28, 63]. However global depletion of CTCF failed to reactivate Type III expression program in Type I latency cells, and CTCF binding was not universally reduced at Cp in all cell types tested [51, 64]. These findings suggest that CTCF binding at Cp may not be a simple blocker of OriP-enhancer function, but may play a more complex role in regulating latency type gene programming, including the formation of different higher-ordered chromatin structures [51].
CTCF contributes to the three-dimensional organization of genome by promoting long-distance interactions between different DNA regions [60, 65, 66]. Genome-wide analysis of chromatin architecture in human cells revealed that long-distance interactions usually involve enhancers and promoters and correlate with gene expression activation [67, 68]. Three-dimensional analysis of EBV genome revealed that chromatin loops are established between the enhancer region OriP and either the Qp in Type I or the Cp in Type III [69]. The integrity of the CTCF binding sites upstream of Cp and Qp is critical for the formation and the maintenance of these chromatin loops [69] (Fig. 3). However the disruption of the loops did not result in the immediate loss of promoter selection and gene expression [28, 51, 63, 69]. Rather, the loss of Qp expression required additional time for its eventual silencing by DNA methylation. These observations suggest that EBV promoter selection and latency switching are hierarchically organized processes regulated by chromatin composition, chromatin folding, and transcription factor binding at key regulatory regions, including OriP. At present we do not know how CTCF binding sites form selective chromatin loops in one type of latency but not in another.
Figure 3. A DNA Looping Function of CTCF at OriP and Cp or Qp.
(Left panel) During Type I latency OriP (orange) forms a loop with the active Qp (green arrows) that is mediated by CTCF-cohesin binding sites. (Right panel) During Type III latency OriP forms a loop with Cp that depends partly on an intervening CTCF binding site. We envision that DNA looping to OriP recruits transcriptional promoters to an active chromatin domain.
The association between CTCF and other cellular or viral factors may also contribute to alternative long-range interactions by stabilizing specific chromatin loops. CTCF can interact with members of the cohesin subunits SMC1, SMC3 and RAD21 [70-72]. The cohesins have essential roles in sister-chromatid cohesion and transcription regulation, and function by forming a ring structure that can link different molecules of DNA [73-75]. Interestingly, cohesins colocalize with some, but not all of the CTCF binding sites, suggesting that at least some CTCF sites have specialized DNA looping functions[26, 62]. Recently, the knockdown of cohesin subunits in Type III latently infected cells resulted in a reduction of LMP1 and LMP2 expression and in the disruption of the long-range interaction between OriP and the LMP1/LMP2 promoter [26]. These findings suggest that a complex network of interactions regulates EBV gene expression. Understanding how these long-range interactions are regulated will expand our knowledge of the epigenetic factors that control EBV gene expression during infection and carcinogenesis.
OriP as a chromosome organizer of EBV
One common epigenetic feature of Types I and III latency is the enrichment of euchromatic marks surrounding the origin of plasmid replication (OriP) and RNA pol III EBER transcripts [17, 26, 29, 76]. The chromatin structure of OriP is thought to be critical for the correct function of OriP as origin of replication, but it is also likely to be important for its function as a transcriptional enhancer of Cp and LMP1/LMP2 in Type III latency. EBNA1 binds to OriP in all latency types and EBNA1 can affect nucleosome phasing and DNA conformation at the FR region of OriP [77]. OriP also interacts with the chromatin remodeling protein SNF2h [76] and components of the origin recognition complex (ORC) that associate with chromatin modifying proteins including the histone acetylase HBO1 and the heterochromatin protein 1 (HP1) [78, 79]. How these factors influence OriP chromatin structure and histone modification patterns, and how these may regulate latency type transcription remains an important area of investigation.
Recent studies have implicated OriP as a central hub in long-range DNA interaction [26, 69]. Long-range interactions between DNA regulatory elements occur frequently in higher eukaryotes and play important roles in gene regulation. Recently genome-wide analysis of the spatial organization in different human cell lines identified more than 1000 long-range interactions between TSS and regulatory elements, including enhancers, promoters and CTCF-binding sites [67]. The spatial analysis of EBV genome showed that OriP can establish long-range interactions with several latency promoters including Cp and LMP1/2 promoters in Type III and Qp in Type I, supporting the idea that OriP is essential for viral promoter activation during latency. The family of repeats (FR) region of OriP has been shown to function as an enhancer of the Cp transcription [80-84]. The three-dimensional organization of EBV episomes suggest that OriP can also function as an enhancer of LMPs in Type III, and Qp in Type I latency [26, 69]. 3C studies indicate that OriP can physically interact with these promoter elements with the intervening DNA looping out, indicating that different episome conformations exist for different latency types. In these studies, OriP appears to have a central organizing role for EBV chromatin structure (Fig. 4).
Figure 4. A Chromatin Hub Function of OriP.
OriP can function as a transcriptional enhancer for Cp and LMP1p in Type III latency. OriP interacts with EBNA1, ORC, and SNF2h chromatin remodeling factors. OriP can form stable loops with Cp and LMP1 mediated by CTCF and cohesins that bind in close proximity to these target promoters forming a viral Locus Control Region (LCR). Elevated H3K4me3 at OriP provides an Active Chromatin Hub (ACH).
The region encompassing the LMP1/2 promoter, EBERs transcription units, and Cp promoter has been proposed to function as a locus control region (LCR) in analogy with cellular β-globin LCR [52, 85, 86]. LCRs are characterized by a strong-enhancer activity, initiation sites for DNA replication, chromatin domain-opening activity, and enrichment for open chromatin marks such as H3Ac and H4Ac [87]. The OriP region of EBV shares most of the LCR properties including enhancer activity and initiation of DNA replication [85]. Cellular LCRs can engage in chromatin looping with the linked genes to form an active chromatin hub (ACH) [88]. OriP can serve as an ACH by mediating multiple interactions with viral and possibly cellular promoter regions. The DS and the FR elements of OriP can also form a DNA loop mediated by EBNA1, suggesting that the spatial organization of OriP involves multiple and overlapping DNA interactions [89]. As for the β-globin ACH [66], CTCF binding sites have been implicated in mediating multiple and overlapping long-range interactions [28, 62]. Based on these observation we suggest that the region encompassing OriP folds to form an ACH and LCR that coordinates the latency type promoter selection and transcription programming.
EBV as an epigenetic regulator of the host chromosome
EBV latent episomes and latency gene products can also influence the epigenetic state of the host genome (summarized in Table 1). For example, EBNA2 interacts with host cell sequence-specific binding factors, like RBP-jK and Pu.1, and transcriptional coactivators, like p300, CBP and PCAF, to modulate both viral and host gene expression [90]. These transcriptional co-activators are histone acetyltransferases (HAT) [91-93] and allow EBNA2 to direct epigenetic reprogramming of the viral and host chromosome. EBNA2 interacts also with hSNF5/In, a member of the Swi/Snf family, which is involved in the nucleosome remodeling [94]. EBNA2 can also recruit the RNA polymerase II elongation factor pTEFb to facilitate the elongation of the large polycistronic EBNA transcript initiating from Cp, an event that can alter local and global chromatin structure of target genes [95]. Presumably, EBNA2 modulates host chromosome sites in a similar fashion.
Table 1. Summary of EBV encoded genes that affect host cell epigenetic regulation.
| Viral Gene | Effect | Reference: |
|---|---|---|
| EBNA1 | Altering DNA methylation | 104 |
| Altering telomere functions | 105 | |
| Altering nucleosome phasing | 111 | |
| EBNA2 | Interacting with HAT and remodeling viral and host epigenome | 91-93 |
| Interacting with hSNF5/In and remodeling nucleosomes | 94 | |
| EBNA3C | Recruiting PRC and repressing host tumor suppressors | 96 |
| LMP1 | Activating DNMT1through JNK-AP-1 signaling | 50, 101 |
| LMP2A | Activating DNMT1 through STAT3 signaling | 102 |
| BART | Targeting cellular genes involved in host epigenetic programming | 103 |
EBNA3C has been shown to repress host tumor suppressor genes through the recruitment of polycomb complex and the formation of histone H3K27me3 [96]. The polycomb repressive complex (PRC) participates in the formation of heterochromatin on developmentally regulated genes, and functions as an important modulator of tumor suppressor gene activity in human carcinogenesis [97]. Ezh2 is a member of the PRC2 complex and can also function in the repression of the EBV genome during latency. Ezh2 binds to the Zta promoter in some latency types to catalyze H3K27me3 associated transcription silencing [31]. It is not yet known whether EBNA3C is responsible for the epigenetic silencing of the EBV genome, in addition to its repression of select genes in the host chromosome.
EBV latent infection has also been linked to the increase DNA methylation of host tumor suppressor genes, especially in nasopharyngeal carcinoma (NPC) [98] and gastric carcinomas (GC) [99, 100]. The viral gene products and mechanisms responsible for site selective DNA methylation of host tumor suppressor genes are not completely clear. LMP1 can activate DNMT1 through the JNK-AP-1 signal transduction pathway in NPC [101], while LMP2A can induce DNMT1 activation in gastric carcinoma through STAT3 signaling [102]. EBV encoded miRNAs are highly elevated in epithelial carcinomas and may target cellular genes important for host epigenetic programming, including DNA methylation patterning [103]. EBNA1 has been implicated in altering DNA methylation patterns at the EBV genomes [104], and can bind to the host cell chromosome at specific sites, but has not yet been shown to affect host cell DNA methylation. Identifying the viral factors and the mechanisms controlling site specific DNA methylation will be necessary for understanding the mechanism of EBV carcinogenesis in epithelial cell carcinomas.
EBV latent infection may remodel the host chromosome structures. Recent studies have shown that immortalization of primary B-lymphocytes alters host chromosome telomeres [105]. Telomere associated DNA damage signals are linked to changes in telomere end-protection and chromatin structural alterations. Ectopic expression of EBNA1 alone was sufficient to produce telomere dysfunction in transformed B-cells, indicating the EBNA1 contributes to telomere dysfunction during EBV latent infection [106]. EBNA1 can bind to numerous sites on the host chromosome [107-110] and may alter chromatin structure or nucleosome positioning at those sites [111]. On the EBV genome, EBNA1 can alter the nucleosome positioning and mediate DNA loop formation [112, 113], so it would not be unexpected to induce similar effects at EBNA1 binding sites on the host-chromosome. Recently it has been demonstrated that EBNA1 binding to the host genome promotes a global change in chromatin organization that results in more open chromatin structure [114]. EBNA1 binding to host chromosomes can alter host cell gene expression [115], but may also function to select chromosome attachment sites for viral episome tethering during mitosis. Thus, EBNA1 may function to alter host chromatin structure to favor viral genome tethering to euchromatic regions of the host chromosome.
Outlook
Epigenetic mechanisms provide a complex and robust level of control of gene expression and genome organization. For mobile genetic elements, like DNA tumor viruses, epigenetic mechanisms play a central role in the rapid assembly and disassembly of viral genomes into functional chromosome-like structures. This is especially significant for EBV, which adopts a variety of gene expression programs and persist in diverse cell types. Epigenetic modifications drive and stabilize these different gene expression programs, and are essential for maintaining a persistent infection in dividing cells. How these epigenetic controls are coordinated with host cell biology and environmental factors remains an important area of future research. Recent advances in deep-sequencing methods and genome-wide bioinformatics analyses have revealed new insights into the complex epigenetic regulation of viral and cellular genomes. These studies show a dynamic interplay of histone and DNA modifications, a complex patterning of transcription factor binding, nucleosome positioning, and chromatin boundaries, and a regulatory role for higher-order chromatin structures and host-chromosome attachments. Deciphering the epigenetic code of cellular and viral genomes will require a more complete understanding of the molecular “writers”, “readers”, and “erasers” and how they are orchestrated to function in a highly coordinated and integrated process. Identifying the epigenetic and systems features that control EBV infection and latency will undoubtedly reveal important new insights into the mechanisms that control viral persistence and pathogenesis, and may provide new therapeutic targets for the treatment of EBV-positive tumors and associated disease.
Acknowledgments
This work was supported by a R00AI099153 award from the National Institute of Allergy and Infectious Diseases to I.T., and R01DE017336 and R01CA093606 to PML.
Footnotes
Conflict of Interest statement: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Italo Tempera, Email: Tempera@temple.edu, The Fels Institute, Department of Microbiology and Immunology, Temple School of Medicine, Philadelphia, PA 19140.
Paul M. Lieberman, Email: Lieberman@wistar.org, The Wistar Institute, Philadelphia, PA 19104.
References
- 1.Kieff E. Epstein-Barr Virus and its replication. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 2.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–68. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 3.Howlader NNA, Krapcho M, Neyman N, Aminou R, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations) April 2012. National Cancer Institute; Bethesda, MD: 2011. [Google Scholar]
- 4.Babcock GJ, Hochberg D, Thorley-Lawson AD. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity. 2000;13:497–506. doi: 10.1016/s1074-7613(00)00049-2. [DOI] [PubMed] [Google Scholar]
- 5.Rowe M, Lear AL, Croom-Carter D, Davies AH, Rickinson AB. Three pathways of Epstein-Barr virus gene activation from EBNA1-positive latency in B lymphocytes. J Virol. 1992;66:122–31. doi: 10.1128/jvi.66.1.122-131.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350:1328–37. doi: 10.1056/NEJMra032015. [DOI] [PubMed] [Google Scholar]
- 7.Qu L, Rowe DT. Epstein-Barr virus latent gene expression in uncultured peripheral blood lymphocytes. J Virol. 1992;66:3715–24. doi: 10.1128/jvi.66.6.3715-3724.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tierney RJ, Steven N, Young LS, Rickinson AB. Epstein-Barr virus latency in blood mononuclear cells: analysis of viral gene transcription during primary infection and in the carrier state. J Virol. 1994;68:7374–85. doi: 10.1128/jvi.68.11.7374-7385.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woisetschlaeger M, Yandava CN, Furmanski LA, Strominger JL, Speck SH. Promoter switching in Epstein-Barr virus during the initial stages of infection of B lymphocytes. Proc Natl Acad Sci U S A. 1990;87:1725–9. doi: 10.1073/pnas.87.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nonkwelo C, Skinner J, Bell A, Rickinson A, Sample J. Transcription start sites downstream of the Epstein-Barr virus (EBV) Fp promoter in early-passage Burkitt lymphoma cells define a fourth promoter for expression of the EBV EBNA-1 protein. J Virol. 1996;70:623–7. doi: 10.1128/jvi.70.1.623-627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tierney R, Kirby H, Nagra J, Rickinson A, Bell A. The Epstein-Barr virus promoter initiating B-cell transformation is activated by RFX proteins and the B-cell-specific activator protein BSAP/Pax5. J Virol. 2000;74:10458–67. doi: 10.1128/jvi.74.22.10458-10467.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes DJ, Dickerson CA, Shaner MS, Sample CE, Sample JT. trans-Repression of protein expression dependent on the Epstein-Barr virus promoter Wp during latency. J Virol. 2011;85:11435–47. doi: 10.1128/JVI.05158-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tempera I, Lieberman PM. Chromatin organization of gammaherpesvirus latent genomes. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbagrm.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishikawa J, Kis LL, Liu A, Zhang X, Takahara M, Bandobashi K, et al. Upregulation of LMP1 expression by histone deacetylase inhibitors in an EBV carrying NPC cell line. Virus Genes. 2004;28:121–8. doi: 10.1023/B:VIRU.0000012268.35297.ff. [DOI] [PubMed] [Google Scholar]
- 15.Masucci MG, Contreras-Salazar B, Ragnar E, Falk K, Minarovits J, Ernberg I, et al. 5-Azacytidine up regulates the expression of Epstein-Barr virus nuclear antigen 2 (EBNA-2) through EBNA-6 and latent membrane protein in the Burkitt's lymphoma line rael. J Virol. 1989;63:3135–41. doi: 10.1128/jvi.63.7.3135-3141.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dyson PJ, Farrell PJ. Chromatin structure of Epstein-Barr virus. J Gen Virol. 1985;66(Pt 9):1931–40. doi: 10.1099/0022-1317-66-9-1931. [DOI] [PubMed] [Google Scholar]
- 17.Wensing B, Stuhler A, Jenkins P, Hollyoake M, Karstegl CE, Farrell PJ. Variant chromatin structure of the oriP region of Epstein-Barr virus and regulation of EBER1 expression by upstream sequences and oriP. J Virol. 2001;75:6235–41. doi: 10.1128/JVI.75.13.6235-6241.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woellmer A, Hammerschmidt W. Epstein-Barr virus and host cell methylation: regulation of latency, replication and virus reactivation. Curr Opin Virol. 2013;3:260–5. doi: 10.1016/j.coviro.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–80. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 20.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–5. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 21.Birney E, Stamatoyannopoulos JA, Dutta A, Guigo R, Gingeras TR, Margulies EH, et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet. 2007;39:311–8. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- 23.Peters AH, Mermoud JE, O'Carroll D, Pagani M, Schweizer D, Brockdorff N, et al. Histone H3 lysine 9 methylation is an epigenetic imprint of facultative heterochromatin. Nat Genet. 2002;30:77–80. doi: 10.1038/ng789. [DOI] [PubMed] [Google Scholar]
- 24.Mermoud JE, Popova B, Peters AH, Jenuwein T, Brockdorff N. Histone H3 lysine 9 methylation occurs rapidly at the onset of random X chromosome inactivation. Curr Biol. 2002;12:247–51. doi: 10.1016/s0960-9822(02)00660-7. [DOI] [PubMed] [Google Scholar]
- 25.Cao R, Zhang Y. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr Opin Genet Dev. 2004;14:155–64. doi: 10.1016/j.gde.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Arvey A, Tempera I, Tsai K, Chen HS, Tikhmyanova N, Klichinsky M, et al. An atlas of the Epstein-Barr virus transcriptome and epigenome reveals host-virus regulatory interactions. Cell Host Microbe. 2012;12:233–45. doi: 10.1016/j.chom.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerle B, Koroknai A, Fejer G, Bakos A, Banati F, Szenthe K, et al. Acetylated histone H3 and H4 mark the upregulated LMP2A promoter of Epstein-Barr virus in lymphoid cells. J Virol. 2007;81:13242–7. doi: 10.1128/JVI.01396-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tempera I, Wiedmer A, Dheekollu J, Lieberman PM. CTCF prevents the epigenetic drift of EBV latency promoter Qp. PLoS Pathog. 2010;6:e1001048. doi: 10.1371/journal.ppat.1001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Day L, Chau CM, Nebozhyn M, Rennekamp AJ, Showe M, Lieberman PM. Chromatin profiling of Epstein-Barr virus latency control region. J Virol. 2007;81:6389–401. doi: 10.1128/JVI.02172-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arvey A, Tempera I, Lieberman PM. Interpreting the Epstein-Barr Virus (EBV) epigenome using high-throughput data. Viruses. 2013;5:1042–54. doi: 10.3390/v5041042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murata T, Kondo Y, Sugimoto A, Kawashima D, Saito S, Isomura H, et al. Epigenetic histone modification of Epstein-Barr virus BZLF1 promoter during latency and reactivation in Raji cells. J Virol. 2012;86:4752–61. doi: 10.1128/JVI.06768-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramasubramanyan S, Osborn K, Flower K, Sinclair AJ. Dynamic chromatin environment of key lytic cycle regulatory regions of the Epstein-Barr virus genome. J Virol. 2012;86:1809–19. doi: 10.1128/JVI.06334-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toth Z, Brulois K, Jung JU. The chromatin landscape of Kaposi's sarcoma-associated herpesvirus. Viruses. 2013;5:1346–73. doi: 10.3390/v5051346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toth Z, Maglinte DT, Lee SH, Lee HR, Wong LY, Brulois KF, et al. Epigenetic analysis of KSHV latent and lytic genomes. PLoS Pathog. 2010;6:e1001013. doi: 10.1371/journal.ppat.1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin B, Tao Q, Peng J, Soo HM, Wu W, Ying J, et al. DNA methyltransferase 3B (DNMT3B) mutations in ICF syndrome lead to altered epigenetic modifications and aberrant expression of genes regulating development, neurogenesis and immune function. Hum Mol Genet. 2008;17:690–709. doi: 10.1093/hmg/ddm341. [DOI] [PubMed] [Google Scholar]
- 36.Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6:597–610. doi: 10.1038/nrg1655. [DOI] [PubMed] [Google Scholar]
- 37.Gopalakrishnan S, Van Emburgh BO, Robertson KD. DNA methylation in development and human disease. Mutat Res. 2008;647:30–8. doi: 10.1016/j.mrfmmm.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robertson KD, Manns A, Swinnen LJ, Zong JC, Gulley ML, Ambinder RF. CpG methylation of the major Epstein-Barr virus latency promoter in Burkitt's lymphoma and Hodgkin's disease. Blood. 1996;88:3129–36. [PubMed] [Google Scholar]
- 39.Paulson EJ, Fingeroth JD, Yates JL, Speck SH. Methylation of the EBV genome and establishment of restricted latency in low-passage EBV-infected 293 epithelial cells. Virology. 2002;299:109–21. doi: 10.1006/viro.2002.1457. [DOI] [PubMed] [Google Scholar]
- 40.Robertson KD, Ambinder RF. Methylation of the Epstein-Barr virus genome in normal lymphocytes. Blood. 1997;90:4480–4. [PubMed] [Google Scholar]
- 41.Kintner C, Sugden B. Conservation and progressive methylation of Epstein-Barr viral DNA sequences in transformed cells. J Virol. 1981;38:305–16. doi: 10.1128/jvi.38.1.305-316.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jansson A, Masucci M, Rymo L. Methylation of discrete sites within the enhancer region regulates the activity of the Epstein-Barr virus BamHI W promoter in Burkitt lymphoma lines. J Virol. 1992;66:62–9. doi: 10.1128/jvi.66.1.62-69.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bakos A, Banati F, Koroknai A, Takacs M, Salamon D, Minarovits-Kormuta S, et al. High-resolution analysis of CpG methylation and in vivo protein-DNA interactions at the alternative Epstein-Barr virus latency promoters Qp and Cp in the nasopharyngeal carcinoma cell line C666-1. Virus Genes. 2007;35:195–202. doi: 10.1007/s11262-007-0095-y. [DOI] [PubMed] [Google Scholar]
- 44.Salamon D, Takacs M, Schwarzmann F, Wolf H, Minarovits J, Niller HH. High-resolution methylation analysis and in vivo protein-DNA binding at the promoter of the viral oncogene LMP2A in B cell lines carrying latent Epstein-Barr virus genomes. Virus Genes. 2003;27:57–66. doi: 10.1023/a:1025124519068. [DOI] [PubMed] [Google Scholar]
- 45.Paulson EJ, Speck SH. Differential methylation of Epstein-Barr virus latency promoters facilitates viral persistence in healthy seropositive individuals. J Virol. 1999;73:9959–68. doi: 10.1128/jvi.73.12.9959-9968.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lehnertz B, Ueda Y, Derijck AA, Braunschweig U, Perez-Burgos L, Kubicek S, et al. Suv39h-mediated histone H3 lysine 9 methylation directs DNA methylation to major satellite repeats at pericentric heterochromatin. Curr Biol. 2003;13:1192–200. doi: 10.1016/s0960-9822(03)00432-9. [DOI] [PubMed] [Google Scholar]
- 47.Takacs M, Salamon D, Myohanen S, Li H, Segesdi J, Ujvari D, et al. Epigenetics of latent Epstein-Barr virus genomes: high resolution methylation analysis of the bidirectional promoter region of latent membrane protein 1 and 2B genes. Biol Chem. 2001;382:699–705. doi: 10.1515/BC.2001.083. [DOI] [PubMed] [Google Scholar]
- 48.Falk KI, Szekely L, Aleman A, Ernberg I. Specific methylation patterns in two control regions of Epstein-Barr virus latency: the LMP-1-coding upstream regulatory region and an origin of DNA replication (oriP) J Virol. 1998;72:2969–74. doi: 10.1128/jvi.72.4.2969-2974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tao Q, Robertson KD, Manns A, Hildesheim A, Ambinder RF. The Epstein-Barr virus major latent promoter Qp is constitutively active, hypomethylated, and methylation sensitive. J Virol. 1998;72:7075–83. doi: 10.1128/jvi.72.9.7075-7083.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leonard S, Wei W, Anderton J, Vockerodt M, Rowe M, Murray PG, et al. Epigenetic and transcriptional changes which follow Epstein-Barr virus infection of germinal center B cells and their relevance to the pathogenesis of Hodgkin's lymphoma. J Virol. 2011;85:9568–77. doi: 10.1128/JVI.00468-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hughes DJ, Marendy EM, Dickerson CA, Yetming KD, Sample CE, Sample JT. Contributions of CTCF and DNA methyltransferases DNMT1 and DNMT3B to Epstein-Barr virus restricted latency. J Virol. 2012;86:1034–45. doi: 10.1128/JVI.05923-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chau CM, Lieberman PM. Dynamic chromatin boundaries delineate a latency control region of Epstein-Barr virus. J Virol. 2004;78:12308–19. doi: 10.1128/JVI.78.22.12308-12319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chevallier-Greco A, Manet E, Chavrier P, Mosnier C, Daillie J, Sergeant A. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 1986;5:3243–9. doi: 10.1002/j.1460-2075.1986.tb04635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zalani S, Holley-Guthrie E, Kenney S. Epstein-Barr viral latency is disrupted by the immediate-early BRLF1 protein through a cell-specific mechanism. Proc Natl Acad Sci U S A. 1996;93:9194–9. doi: 10.1073/pnas.93.17.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramasubramanyan S, Kanhere A, Osborn K, Flower K, Jenner RG, Sinclair AJ. Genome-wide analyses of Zta binding to the Epstein-Barr virus genome reveals interactions in both early and late lytic cycles and an epigenetic switch leading to an altered binding profile. J Virol. 2012;86:12494–502. doi: 10.1128/JVI.01705-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bergbauer M, Kalla M, Schmeinck A, Gobel C, Rothbauer U, Eck S, et al. CpG-methylation regulates a class of Epstein-Barr virus promoters. PLoS Pathog. 2010;6:e1001114. doi: 10.1371/journal.ppat.1001114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhende PM, Seaman WT, Delecluse HJ, Kenney SC. The EBV lytic switch protein, Z, preferentially binds to and activates the methylated viral genome. Nat Genet. 2004;36:1099–104. doi: 10.1038/ng1424. [DOI] [PubMed] [Google Scholar]
- 58.Yu X, Wang Z, Mertz JE. ZEB1 regulates the latent-lytic switch in infection by Epstein-Barr virus. PLoS Pathog. 2007;3:e194. doi: 10.1371/journal.ppat.0030194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Filippova GN, Fagerlie S, Klenova EM, Myers C, Dehner Y, Goodwin G, et al. An exceptionally conserved transcriptional repressor, CTCF, employs different combinations of zinc fingers to bind diverged promoter sequences of avian and mammalian c-myc oncogenes. Mol Cell Biol. 1996;16:2802–13. doi: 10.1128/mcb.16.6.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Phillips JE, Corces VG. CTCF: master weaver of the genome. Cell. 2009;137:1194–211. doi: 10.1016/j.cell.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ohlsson R, Renkawitz R, Lobanenkov V. CTCF is a uniquely versatile transcription regulator linked to epigenetics and disease. Trends Genet. 2001;17:520–7. doi: 10.1016/s0168-9525(01)02366-6. [DOI] [PubMed] [Google Scholar]
- 62.Holdorf MM, Cooper SB, Yamamoto KR, Miranda JJ. Occupancy of chromatin organizers in the Epstein-Barr virus genome. Virology. 2011;415:1–5. doi: 10.1016/j.virol.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chau CM, Zhang XY, McMahon SB, Lieberman PM. Regulation of Epstein-Barr virus latency type by the chromatin boundary factor CTCF. J Virol. 2006;80:5723–32. doi: 10.1128/JVI.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salamon D, Banati F, Koroknai A, Ravasz M, Szenthe K, Bathori Z, et al. Binding of CCCTC-binding factor in vivo to the region located between Rep* and the C promoter of Epstein-Barr virus is unaffected by CpG methylation and does not correlate with Cp activity. J Gen Virol. 2009;90:1183–9. doi: 10.1099/vir.0.007344-0. [DOI] [PubMed] [Google Scholar]
- 65.Majumder P, Boss JM. CTCF controls expression and chromatin architecture of the human major histocompatibility complex class II locus. Mol Cell Biol. 2010;30:4211–23. doi: 10.1128/MCB.00327-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Splinter E, Heath H, Kooren J, Palstra RJ, Klous P, Grosveld F, et al. CTCF mediates long-range chromatin looping and local histone modification in the beta-globin locus. Genes Dev. 2006;20:2349–54. doi: 10.1101/gad.399506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sanyal A, Lajoie BR, Jain G, Dekker J. The long-range interaction landscape of gene promoters. Nature. 2012;489:109–13. doi: 10.1038/nature11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fraser P, Bickmore W. Nuclear organization of the genome and the potential for gene regulation. Nature. 2007;447:413–7. doi: 10.1038/nature05916. [DOI] [PubMed] [Google Scholar]
- 69.Tempera I, Klichinsky M, Lieberman PM. EBV latency types adopt alternative chromatin conformations. PLoS Pathog. 2011;7:e1002180. doi: 10.1371/journal.ppat.1002180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rubio ED, Reiss DJ, Welcsh PL, Disteche CM, Filippova GN, Baliga NS, et al. CTCF physically links cohesin to chromatin. Proc Natl Acad Sci U S A. 2008;105:8309–14. doi: 10.1073/pnas.0801273105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stedman W, Kang H, Lin S, Kissil JL, Bartolomei MS, Lieberman PM. Cohesins localize with CTCF at the KSHV latency control region and at cellular c-myc and H19/Igf2 insulators. EMBO J. 2008;27:654–66. doi: 10.1038/emboj.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wendt KS, Yoshida K, Itoh T, Bando M, Koch B, Schirghuber E, et al. Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature. 2008;451:796–801. doi: 10.1038/nature06634. [DOI] [PubMed] [Google Scholar]
- 73.Nasmyth K, Haering CH. The structure and function of SMC and kleisin complexes. Annu Rev Biochem. 2005;74:595–648. doi: 10.1146/annurev.biochem.74.082803.133219. [DOI] [PubMed] [Google Scholar]
- 74.Gruber S, Haering CH, Nasmyth K. Chromosomal cohesin forms a ring. Cell. 2003;112:765–77. doi: 10.1016/s0092-8674(03)00162-4. [DOI] [PubMed] [Google Scholar]
- 75.Zhang N, Kuznetsov SG, Sharan SK, Li K, Rao PH, Pati D. A handcuff model for the cohesin complex. J Cell Biol. 2008;183:1019–31. doi: 10.1083/jcb.200801157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou J, Chau CM, Deng Z, Shiekhattar R, Spindler MP, Schepers A, et al. Cell cycle regulation of chromatin at an origin of DNA replication. EMBO J. 2005;24:1406–17. doi: 10.1038/sj.emboj.7600609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Avolio-Hunter TM, Frappier L. EBNA1 efficiently assembles on chromatin containing the Epstein-Barr virus latent origin of replication. Virology. 2003;315:398–408. doi: 10.1016/s0042-6822(03)00561-0. [DOI] [PubMed] [Google Scholar]
- 78.Iizuka M, Stillman B. Histone acetyltransferase HBO1 interacts with the ORC1 subunit of the human initiator protein. J Biol Chem. 1999;274:23027–34. doi: 10.1074/jbc.274.33.23027. [DOI] [PubMed] [Google Scholar]
- 79.Pak DT, Pflumm M, Chesnokov I, Huang DW, Kellum R, Marr J, et al. Association of the origin recognition complex with heterochromatin and HP1 in higher eukaryotes. Cell. 1997;91:311–23. doi: 10.1016/s0092-8674(00)80415-8. [DOI] [PubMed] [Google Scholar]
- 80.Altmann M, Pich D, Ruiss R, Wang J, Sugden B, Hammerschmidt W. Transcriptional activation by EBV nuclear antigen 1 is essential for the expression of EBV's transforming genes. Proc Natl Acad Sci U S A. 2006;103:14188–93. doi: 10.1073/pnas.0605985103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nilsson T, Zetterberg H, Wang YC, Rymo L. Promoter-proximal regulatory elements involved in oriP-EBNA1-independent and -dependent activation of the Epstein-Barr virus C promoter in B-lymphoid cell lines. J Virol. 2001;75:5796–811. doi: 10.1128/JVI.75.13.5796-5811.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Puglielli MT, Woisetschlaeger M, Speck SH. oriP is essential for EBNA gene promoter activity in Epstein-Barr virus-immortalized lymphoblastoid cell lines. J Virol. 1996;70:5758–68. doi: 10.1128/jvi.70.9.5758-5768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reisman D, Sugden B. trans activation of an Epstein-Barr viral transcriptional enhancer by the Epstein-Barr viral nuclear antigen 1. Mol Cell Biol. 1986;6:3838–46. doi: 10.1128/mcb.6.11.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sugden B, Warren N. A promoter of Epstein-Barr virus that can function during latent infection can be transactivated by EBNA-1, a viral protein required for viral DNA replication during latent infection. J Virol. 1989;63:2644–9. doi: 10.1128/jvi.63.6.2644-2649.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takacs M, Banati F, Koroknai A, Segesdi J, Salamon D, Wolf H, et al. Epigenetic regulation of latent Epstein-Barr virus promoters. Biochim Biophys Acta. 2010;1799:228–35. doi: 10.1016/j.bbagrm.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 86.Grosveld F, van Assendelft GB, Greaves DR, Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987;51:975–85. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- 87.Li Q, Peterson KR, Fang X, Stamatoyannopoulos G. Locus control regions. Blood. 2002;100:3077–86. doi: 10.1182/blood-2002-04-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tolhuis B, Palstra RJ, Splinter E, Grosveld F, de Laat W. Looping and interaction between hypersensitive sites in the active beta-globin locus. Mol Cell. 2002;10:1453–65. doi: 10.1016/s1097-2765(02)00781-5. [DOI] [PubMed] [Google Scholar]
- 89.Frappier L, O'Donnell M. Epstein-Barr nuclear antigen 1 mediates a DNA loop within the latent replication origin of Epstein-Barr virus. Proc Natl Acad Sci U S A. 1991;88:10875–9. doi: 10.1073/pnas.88.23.10875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang L, Grossman SR, Kieff E. Epstein-Barr virus nuclear protein 2 interacts with p300, CBP, and PCAF histone acetyltransferases in activation of the LMP1 promoter. Proc Natl Acad Sci U S A. 2000;97:430–5. doi: 10.1073/pnas.97.1.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bannister AJ, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–3. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 92.Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–9. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 93.Blanco JC, Minucci S, Lu J, Yang XJ, Walker KK, Chen H, et al. The histone acetylase PCAF is a nuclear receptor coactivator. Genes Dev. 1998;12:1638–51. doi: 10.1101/gad.12.11.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu DY, Kalpana GV, Goff SP, Schubach WH. Epstein-Barr virus nuclear protein 2 (EBNA2) binds to a component of the human SNF-SWI complex, hSNF5/Ini1. J Virol. 1996;70:6020–8. doi: 10.1128/jvi.70.9.6020-6028.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Palermo RD, Webb HM, West MJ. RNA polymerase II stalling promotes nucleosome occlusion and pTEFb recruitment to drive immortalization by Epstein-Barr virus. PLoS Pathog. 2011;7:e1002334. doi: 10.1371/journal.ppat.1002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Paschos K, Allday MJ. Epigenetic reprogramming of host genes in viral and microbial pathogenesis. Trends Microbiol. 2010;18:439–47. doi: 10.1016/j.tim.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Valk-Lingbeek ME, Bruggeman SW, van Lohuizen M. Stem cells and cancer; the polycomb connection. Cell. 2004;118:409–18. doi: 10.1016/j.cell.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 98.Macdiarmid J, Stevenson D, Campbell DH, Wilson JB. The latent membrane protein 1 of Epstein-Barr virus and loss of the INK4a locus: paradoxes resolve to cooperation in carcinogenesis in vivo. Carcinogenesis. 2003;24:1209–18. doi: 10.1093/carcin/bgg070. [DOI] [PubMed] [Google Scholar]
- 99.Chong JM, Sakuma K, Sudo M, Ushiku T, Uozaki H, Shibahara J, et al. Global and non-random CpG-island methylation in gastric carcinoma associated with Epstein-Barr virus. Cancer Sci. 2003;94:76–80. doi: 10.1111/j.1349-7006.2003.tb01355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kang GH, Lee S, Kim WH, Lee HW, Kim JC, Rhyu MG, et al. Epstein-barr virus-positive gastric carcinoma demonstrates frequent aberrant methylation of multiple genes and constitutes CpG island methylator phenotype-positive gastric carcinoma. Am J Pathol. 2002;160:787–94. doi: 10.1016/S0002-9440(10)64901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tsai CL, Li HP, Lu YJ, Hsueh C, Liang Y, Chen CL, et al. Activation of DNA methyltransferase 1 by EBV LMP1 Involves c-Jun NH(2)-terminal kinase signaling. Cancer Res. 2006;66:11668–76. doi: 10.1158/0008-5472.CAN-06-2194. [DOI] [PubMed] [Google Scholar]
- 102.Hino R, Uozaki H, Murakami N, Ushiku T, Shinozaki A, Ishikawa S, et al. Activation of DNA methyltransferase 1 by EBV latent membrane protein 2A leads to promoter hypermethylation of PTEN gene in gastric carcinoma. Cancer Res. 2009;69:2766–74. doi: 10.1158/0008-5472.CAN-08-3070. [DOI] [PubMed] [Google Scholar]
- 103.Marquitz AR, Raab-Traub N. The role of miRNAs and EBV BARTs in NPC. Semin Cancer Biol. 2012;22:166–72. doi: 10.1016/j.semcancer.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lin IG, Tomzynski TJ, Ou Q, Hsieh CL. Modulation of DNA binding protein affinity directly affects target site demethylation. Mol Cell Biol. 2000;20:2343–9. doi: 10.1128/mcb.20.7.2343-2349.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kamranvar SA, Chen X, Masucci MG. Telomere dysfunction and activation of alternative lengthening of telomeres in B-lymphocytes infected by Epstein-Barr virus. Oncogene. 2013 doi: 10.1038/onc.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kamranvar SA, Masucci MG. The Epstein-Barr virus nuclear antigen-1 promotes telomere dysfunction via induction of oxidative stress. Leukemia. 2011;25:1017–25. doi: 10.1038/leu.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lu F, Wikramasinghe P, Norseen J, Tsai K, Wang P, Showe L, et al. Genome-wide analysis of host-chromosome binding sites for Epstein-Barr Virus Nuclear Antigen 1 (EBNA1) Virol J. 2010;7:262. doi: 10.1186/1743-422X-7-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Canaan A, Haviv I, Urban AE, Schulz VP, Hartman S, Zhang Z, et al. EBNA1 regulates cellular gene expression by binding cellular promoters. Proc Natl Acad Sci U S A. 2009;106:22421–6. doi: 10.1073/pnas.0911676106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sompallae R, Callegari S, Kamranvar SA, Masucci MG. Transcription profiling of Epstein-Barr virus nuclear antigen (EBNA)-1 expressing cells suggests targeting of chromatin remodeling complexes. PLoS One. 2010;5:e12052. doi: 10.1371/journal.pone.0012052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dresang LR, Vereide DT, Sugden B. Identifying sites bound by Epstein-Barr virus nuclear antigen 1 (EBNA1) in the human genome: defining a position-weighted matrix to predict sites bound by EBNA1 in viral genomes. J Virol. 2009;83:2930–40. doi: 10.1128/JVI.01974-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang S, Frappier L. Nucleosome assembly proteins bind to Epstein-Barr virus nuclear antigen 1 and affect its functions in DNA replication and transcriptional activation. J Virol. 2009;83:11704–14. doi: 10.1128/JVI.00931-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou J, Chau C, Deng Z, Stedman W, Lieberman PM. Epigenetic control of replication origins. Cell Cycle. 2005;4:889–92. doi: 10.4161/cc.4.7.1823. [DOI] [PubMed] [Google Scholar]
- 113.Avolio-Hunter TM, Lewis PN, Frappier L. Epstein-Barr nuclear antigen 1 binds and destabilizes nucleosomes at the viral origin of latent DNA replication. Nucleic Acids Res. 2001;29:3520–8. doi: 10.1093/nar/29.17.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Coppotelli G, Mughal N, Callegari S, Sompallae R, Caja L, Luijsterburg MS, et al. The Epstein-Barr virus nuclear antigen-1 reprograms transcription by mimicry of high mobility group A proteins. Nucleic Acids Res. 2013;41:2950–62. doi: 10.1093/nar/gkt032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gruhne B, Sompallae R, Marescotti D, Kamranvar SA, Gastaldello S, Masucci MG. The Epstein-Barr virus nuclear antigen-1 promotes genomic instability via induction of reactive oxygen species. Proc Natl Acad Sci U S A. 2009;106:2313–8. doi: 10.1073/pnas.0810619106. [DOI] [PMC free article] [PubMed] [Google Scholar]