Abstract
Angiogenesis and metastasis are well recognized as processes fundamental to the development of malignancy. Both processes involve the coordination of multiple cellular and chemical activities through myriad signaling networks, providing a mass of potential targets for therapeutic intervention. This review will focus on one master regulator of cell motility, RAC1, and the existing data with regard to its role in cell motility, including particular roles for tumor angiogenesis and invasion/metastasis. We also emphasize the pre-clinical investigations carried out with RAC1 inhibitors to evaluate the therapeutic potential of this target. Herein we explore potential future directions as well as the challenges of targeting RAC1 in the treatment of cancer. Recent insights at the molecular and cellular levels are paving the way for a more directed and detailed approach to target mechanisms of RAC1 regulating angiogenesis and metastasis. Understanding these mechanisms may provide insight into RAC1 signaling components as alternative therapeutic targets for tumor angiogenesis and metastasis.
Keywords: RAC1, targeted therapeutics, angiogenesis, metastasis, experimental therapeutics
Introduction
RAC1 is a member of the Rho GTPase family, which includes RHO, RAC1, and CDC42. These proteins classically regulate the machinery that controls the assembly and disassembly of cytoskeletal elements (reviewed in (1)). RAC1 activity, as a modulator of the cytoskeleton, is critical for a number of normal cellular activities, including phagocytosis, mesenchymal-like migration, axonal growth, adhesion and differentiation of multiple cell types as well as ROS-mediated cell killing (reviewed in (2)). RAC1 also plays a major role in the moderation of other signaling pathways involved in cellular growth and cell cycle regulation(3), the formation of cell-cell adhesions(4), and the process of contact inhibition(5). These RAC-1-mediated activities appear central to the processes that underlie malignant transformation, including tumorigenesis, angiogenesis, invasion, and metastasis. As such, some have posited that this protein and its partners may prove effective targets for drugs aimed at disrupting these pathways that mediate malignancy.
RAC1 appears to be deregulated in both expression and activity in a variety of tumor cells(6). RAC1 hyper-activation and overexpression seems to correlate well with aggressive growth and other malignant characteristics in several different tumor types. This correlative evidence, coupled with our ever-improving appreciation for the mechanistic role that RAC1 plays in a number of malignancy-related processes has piqued the interest of several groups who have speculated on the plausibility of targeting RAC1 and associated intracellular machinery for the potential anti-tumor effects that manipulating these pathways might have. This review will focus on potential roles of RAC1 in angiogenesis and metastasis and will summarize efforts that have been made to target these pathways. We attempt to present in an organized fashion multiple lines of evidence that illustrate how RAC1 activity contributes to these complex processes and offer an assessment of how we might exploit this target to disrupt the process of malignant progression.
RAC1 Signaling
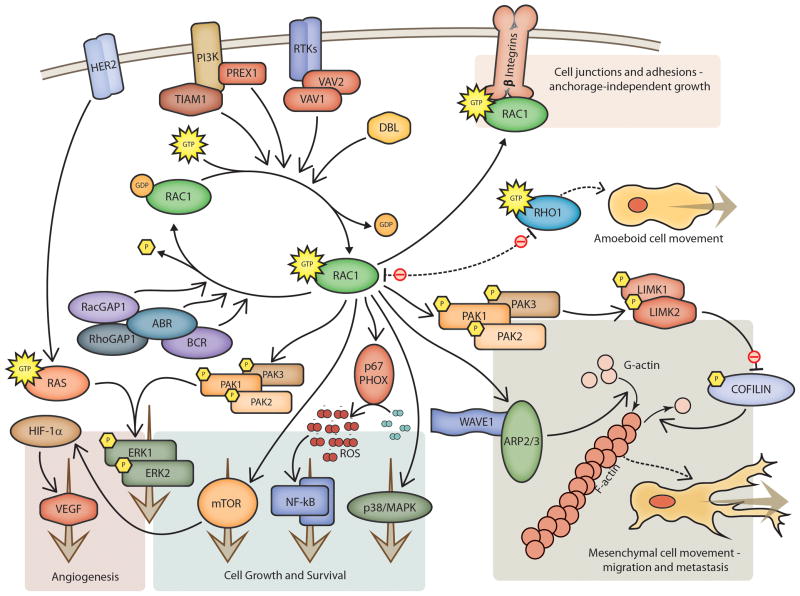
RAC1 signaling pathways play an important role in the pathobiology of various processes that promote tumor progression. Consistent with this, growing evidence from animal studies clearly indicates that RAC1 signaling contributes to tumorigenesis. RAC1 primarily activates p21-activated kinases, such as PAK1, PAK2, and PAK3. PAKs are serine/threonine kinases that phosphorylate and activate actin binding LIM kinases, LIMK1 and LIMK2, which in turn phosphorylate and inactivate cofilin (Figure 1). Cofilin binds actin filaments, reversing the process of polymerization brought on by ARP2/3 activity, converting F-actin filaments into G-actin monomers. By inactivating cofilin, these pathways allow actin filaments to grow(7). RAC1 also causes, together with the adapter protein NCK, dissociation of WAVE1 from its regulatory complex, which stimulates activation of the ARP2/3 complex and promotes actin polymerization(8). RAC1 also appears to interact directly with pathways that regulate proliferation (through the MAP kinase system, especially JNK/p38), the inflammatory response (through interactions with NF-κB), ROS-mediated cell killing (through NADPH oxidase), G1 cell-cycle progression and the formation of cell-cell contacts (reviewed in (9)).
Figure 1. RAC Pathways.
Schematic illustration of RAC signaling pathways and effector functions. Emphasis is given to pathways known to affect tumor-related angiogenesis and metastasis.
Under normal conditions, RAC1 activity is controlled both spatially and temporally by the opposing activities of guanine nucleotide exchange factors (GEFs, reviewed in (10)), which exchange GDP for GTP and activate RAC1, and GTPase activating proteins (GAPs), which stimulate the conversion of bound GTP to GDP and inactivate RAC1. The classical GEF activators of RAC1 include VAV1 and VAV2, DBL, and TIAM1, while the most common GAPs include RhoGAP1, RacGAP1, ABR, and BCR, among others. Further regulation of RAC1 activity comes from the crosstalk that exists between these related signaling pathways. For instance, RAC1 and MYC activity appear to be modulated through a negative feedback co-regulatory loop involving α6β4 integrin and PAK2. Further regulatory input comes from the integration of multiple signals by downstream targets of RAC, as is seen in the modulation of STAT3 activity through LIMK1(11).
RAC1 and Angiogenesis
Tumor angiogenesis is requisite for supplying a tumor with the nutrients and waste exchange that are needed to grow from a microscopic collection of tumor cells to a macroscopic tumor organ. This highly regulated process is orchestrated by the balance of inhibitors and stimulators of endothelial cell proliferation, endothelial cell migration, and capillary formation molecules(12). Antiangiogenic agents have been developed and used for the treatment of many different types of tumors. However, clinical trials have had largely disappointing results, which has raised questions about both the strategies employed in targeting angiogenesis and the ways that these agents might be used more effectively(13). The results of these trials have amplified our understanding of neoangiogenesis and of the complexity of interactions between tumor cells and the surrounding stroma. We know that sprouting angiogenesis and lymphatic-blood vessel segregation require highly coordinated endothelial cell migration, an activity often associated with Rho kinases. As is discussed below, these RAC1-dependent processes help to orchestrate a number of cellular responses, which include, for instance, neoangiogenesis that occurs during wound repair and tissue responses to trauma. A detailed role for RAC1 in tumor-related angiogenesis is emerging and suggests an intimate role in that process as well.
Vasculogenesis and angiogenesis are two distinct processes, one representing vessel formation from undifferentiated precursors while the other describes the process where new vessels form from the preexisting vasculature. There is evidence that RAC1 plays a role in both processes. Endothelial-specific deletion of RAC1 results in mid-gestational embryonic lethality(14). These embryos develop defects of major vessels and demonstrate a complete lack of small-branched vessels within both the embryos and their yolk sacs. The effect seems to result from an inhibition of cell migration, likely mediated through an F-actin-related mechanism(15).
RAC1 regulates a diverse spectrum of cellular functions involved in vascular morphogenesis. Double disruption of CDC42 (Cell division control protein 42 homolog) and RAC1 makes endothelial cells unable to form lumens or tubes and blocks endothelial cell invasion in three-dimensional collagen matrices(16). RAC-mediated mesenchymal migration is important in the initial steps of neo-angiogenesis and the separation of new vasculature into blood and lymph vessels. Endothelial-specific RAC1 knockout mice exhibit hemorrhage and edema, which was traced back to the inability of lymphatic endothelial cells to separate from established vessels and form separate lymphatic structures(17). RAC1 also plays a key role in coordinating the initial formation of cell-cell adhesions(18), which play an important role in the assembly of endothelial cells into vessel structures and the maturation of new vasculature. Microinjection of plasmids containing dominant-negative RAC constructs into human vascular endothelial cells leaves them unable to undergo the morphogenic changes that result in capillary formation, while dominant negative RHO and CDC42 do not affect these same processes(19).
A robust line of experimental evidence supports the critical role that RAC1 plays in tumor angiogenesis. RAC1 knockdown by siRNA treatment of vascular endothelial cells inhibits VEGF-mediated tube formation as well as endothelial cell migration, invasion, and proliferation in vitro(20). This anti-angiogenic activity translates well to in vivo models. Matrigel plugs embedded with the same siRNA constructs also demonstrate reduced angiogenesis. RAC1 knockdown within Neuro2a tumors almost completely inhibited the growth of those tumors in a xenograft model. Microscopic examination of those tumors showed less than half the microvascular density of control tumors. Similar anti-tumor activity was seen with the disruption of upstream signals using si-RNAs directed at VAV2/3. Similar effects were seen when B16 melanoma and Lewis lung carcinoma cells were injected into VAV2/3 deficient host mice. Results of these studies(21) showed similarly decreased growth, increased survival, and reduced micro-vascular density. This effect was shown to result, at least partially, from the impaired function of the VAV2/3-deficient endothelial cells.
There does appear to be some redundancy of the RAC1-mediated functions in some tumor models. D’Amico et al(22) were surprised to find that endothelial-specific knockout of RAC1 did not alter tumor growth or angiogenesis. While the absence of β3 integrin alone caused tumors to grow more rapidly, the same endothelial-specific RAC1 depletion in β3-deficient mice retarded the growth of melanotic tumors to rates similar to FLK-1 knockout animals, which lack VEGF receptor 1. These studies suggest redundant pathways for stimulating angiogenesis: one activated by VEGF that is RAC1 dependent, and another activated by β3 integrin that utilizes pathways which are RAC1-independent. The extent to which this apparent redundancy is relevant to other tumor or host types remains to be seen, though studies summarized above suggest that it is not universal.
This collective literature suggests a pivotal role for RAC1 with its upstream and downstream signaling network in the process of tumor angiogenesis. RAC1 and its related pathways seem to be obvious potential targets for therapies intended for the treatment of numerous human diseases that involve abnormal neovascularization, most notably solid tumors.
RAC1 and Metastasis
Metastasis is a complex multistep process. It involves invasion of local tissues, intra-vasation of cancer cells into blood and lymphatic vessels, transit of cancer cells through these vascular trees, survival in foreign environments, extravasation within distant organs, transformation to micro-metastasis forming small cancer nodules, and finally an invasion within the distant tissues, transforming micro-metastases into macro-metastases(23). Metastasis has a major impact on the morbidity and mortality of cancer patients. Despite this clinical relevance, metastasis remains the most poorly elucidated aspect of carcinogenesis. Understanding the mechanisms of metastasis will require further clarification of the underlying cellular and molecular events that control the metastatic cascade from onset to colonization(24). The cellular processes that control motility and adhesion are critical to multiple steps in the metastatic cascade. As illustrated above, RAC1 plays an integral role in both of these cellular processes. The interactions of cancer cells with the endothelium, stroma, and extracellular matrix largely determine patterns of metastatic spread(25). RAC1 participates intimately in the formation of cell-cell adhesions and so likely plays a role in determining these patterns as well. The epithelial-to-mesenchymal transition that heralds the acquisition of an invasive phenotype involves intracellular machinery that facilitates particular types of cell migration. Where RHO typically coordinates cellular activities associated with amoeboid cell motility, RAC regulates actin cytoskeleton reorganization to form cell surface extensions (lamellipodia) typical of mesenchymal movements. This activity is required for cell migration/invasion during cancer metastasis(26).
Members of the RAC family of small GTPases are key regulators of actin cytoskeletal structures and play integral roles in integrin-mediated adhesion and migration. A number of correlative studies have lent support for a central role of this pathway in a number of the mechanisms of metastasis. Baugher et al 2005 found a direct correlation between metastatic potential and endogenous RAC activity in a panel of metastatic human breast cancer cells(27). RAC1 activity and expression levels of PAK1 were associated with poorly differentiated tumors, local invasion and lymph node metastasis in urothelial carcinoma of the upper urinary tract (28). Most squamous cancers of the head and neck exhibit high levels of GTP-bound RAC1 due to EGFR-based autocrine activation of VAV2. Abrogation of VAV2 activity by RNAi dramatically reduces the migratory and invasive phenotypes that these cells exhibit(29). Glioblastomas and breast cancer cells express high levels of the GEFs TRIO, ECT2, and VAV3, which act through RAC1 to promote growth and metastasis(30). Depletion of these GEFs suppresses cell migration, invasion, and proliferation.
The effects of the proteins in this regulatory network may not simply result from promoting or inhibiting RAC1 activity. For instance, silencing of the D4-GDI (RHO GDP dissociation inhibitor) with RNAi in human breast cancer cell lines that overexpress this inhibitor actually abrogates tumor growth and lung metastasis [30]. D4-GDI associates with RAC1 and RAC3 in these cell lines, but not with other RHO GTPases. The exact mechanism that mediates this phenomenon has not been elucidated, but the loss of D4-GDI inhibition appears to block anchorage-independent growth, restore anoikis, and induce apoptosis[31].
Hepatocellular carcinomas often harbor activating mutations in LMCD1, a downstream target of RAC, that increase lamellipodial protrusion and augment the metastatic virulence of the tumor cells carrying the mutation(31). In other HCC patient samples and cell lines, microRNA-142-3p (miR-142-3p) targets RAC1 expression by binding to the 3′UTR of RAC1(32). Overexpression of miR-142-3p reduces RAC1 mRNA and protein levels and inhibits colony formation, migration and invasion in the HCC cell lines. As miR-142 is frequently downregulated in HCC(33), this demonstrates yet another mechanism by which these cells augment RAC activity to promote malignant transformation.
RAC activity mediates some tissue tropism. For instance, prostate cancers overexpress PREX1, which acts as a PI3K-dependent GEF to activate RAC1(34). The activity of this GEF mediates spontaneous prostate cancer metastasis in vivo(30). This process may occur through RAC1-mediated tight binding to bone marrow endothelial cells, which promotes retraction of endothelial cells and diapedesis/extravasation of the tumors in these capillary beds(35). This results from RAC1 activation of β1 integrin, which allows tight binding to occur.
The interplay between the RAC and RHO systems regulates the dynamic reorganization of the actin cytoskeleton. Some tumor types acquire aberrant regulation within these systems, which allows tumor cells to switch back and forth between amoeboid and mesenchymal-type movement(27, 28). Mesenchymal-type migration in melanoma cells is driven by NEDD9 (a metastasis-related gene) and DOCK3 (a RAC GEF) through the modulation of RAC1 activity(35). PTHrP (parathyroid hormone-related peptide)-stimulated cell migration and invasion in colorectal adenocarcinomas is dependent on RAC1 activity, which is augmented by the increased expression of α6β4 integrin and TIAM1 these cells exhibit(36).
RAC1 and both its upstream activators and downstream effectors therefore appear to modulate numerous critical elements of invasion and metastasis. This has been shown repeatedly over multiple tumor types and at multiple unique points in the RAC1 pathways. The consistency of these effects strengthens the validity of RAC1 as a potentially viable target of efforts to disrupt invasion and metastasis.
Alternative roles for RAC1 in tumorigenesis
RAC1 also helps regulate the activity of the mTORC1 and mTORC2 complexes by anchoring those complexes to particular locations within the actin cytoskeleton in a GTP-independent manner(3). Modulation of cell growth and proliferation has also been seen through RAC1-mediated activation of NFkB, especially in melanoma cells(37). NF2 knockout mouse embryonic fibroblasts (MEFs) exhibit increased RAC1 activity (RAC1 activity is inversely regulated by NF2), loss of contact inhibition, and significantly increased canonical Wnt signaling(5). Transfection of these cells with a dominant negative RAC1 construct abrogates the increased activity of the Wnt pathway and restores contact inhibition. This suggests a central role of the RAC1-NF2 interplay in regulating Wnt signaling and in the process of contact inhibition. It has been shown that RAC1 plays a key role in the initial formation and subsequent strengthening of cell-cell adhesion and the formation of cellular junctions(4). Colorectal cancer cells express an alternatively spliced RAC1b and depend on RAC1b signaling for survival(38).
One consequence of abnormal HER2/neu activity is the generation of autocrine and paracrine growth factors, including TGF-β, which can stimulate the growth not only of the malignant cells which contain amplified HER2, but also of nearby stromal cells that do not. RAC1 mediates the expression of many of these signals (39). Abrogation of this activity by overexpression of a dominant negative RAC1 construct suggests that therapeutic targeting of this pathway may affect not only the malignant cells themselves, but may also have effects on the microenvironment that could impede tumor growth and deter malignant transformation.
RAC1 as a therapeutic target
Several attempts have been made at targeting RAC1 and its regulatory network. While RAC1 and its primary regulators (GEFs and GAPs) are not classically druggable targets, there has been some success in designing drugs that can target their activity. When the activity of a naturally-occurring antibiotic (brefeldin A, Figure 2a) was shown to target the complex formed by the interaction of a G-protein (ARF) with its GEF(40, 41), many took note of the unique way that this small molecule interacted with this transient intermediate complex (Figure 2b,c) and began a search for synthetic compounds that might similarly demonstrate interfacial inhibition with other G-protein-GEF complexes(42).
Figure 2. Brefeldin A, a model for small-molecule inhibition of GTPases.

a) Molecular structure of brefeldin A. b) and c) Crystal structure of brefeldin A (at center) in complex with its target GTPase (ARF, light blue) and GEF (Sec7, dark blue). Images are shown in both b) ribbon diagram and c) a space-filling model. The hydrolyzed GDP is seen at front with the catalytic magnesium ion in green. Brefeldin binds the surface of ARF that interacts with its GEFs and prevents the conformational changes that result in GDP for GTP exchange. Images were generated using Protein Data Bank (PDB) data published by Mossessova et al, manipulated with Jmol software, and rendered using POV-ray.
NSC23766 is a synthetic compound identified using computational screens to identify small molecules that interact with the surface of RAC1 that mediates GEF activation(43) and fits nicely into a surface groove of RAC1 known to be critical for GEF specification (Figure 3a). It effectively inhibits RAC1 binding and activation by the Rac-specific GEFs TRIO or TIAM1 in a concentration-dependent manner without interfering CDC42 or RHOA binding in vitro. It also potently blocks serum- or PDGF-induced RAC1 activation and lamellipodia formation without compromising cellular movement mediated by CDC42 or RHOA. Treatment of cells in culture with NSC23766 blocks invasion and metastasis of multiple different tumor types(44, 45). It has also been shown to block neo-angiogenesis in different disease models(44, 46, 47). While in vivo data remains sparse on these agents, NSC23766 has been used in several small studies to block the dissemination of lymphomas(48, 49). Others showed that pharmacologic blockade of RAC1 activity by NSC23766 can induce cell cycle arrest or apoptosis of different breast cancer cell lines without affecting the growth of normal mammary epithelial cells(50), and preliminary studies suggest that RAC inhibiton might reverse some trastuzumab-resistant phenotypes(45). Small studies have suggested that other RAC1 inhibitors, such as EHT 1864(51) (Figure 3a), can downregulate estrogen-receptor expression in ER+ breast tumors(52).
Figure 3. RAC inhibitors and RAC pathway inhibitors.

Chemical structures for a) RAC inhibitors and b) inhibitors of RAC effector molecules discussed in the text.
While many of the early studies of these agents seem promising, NSC23766 does not have sufficient efficacy to be useful clinically. Utilizing data gained from studies on the complex formed by NSC23766 and RAC1, several new structurally-unrelated compounds were identified in computational screens that also had potential to specifically block RAC-GEF interactions(53), though wet studies on these compounds remain forthcoming. Similarly, Montalvo-Ortiz et al used NSC23766 as a starting point to synthesize a number of related compounds, searching for molecules with greater potency and lower IC50s that would make them more plausible agents for therapeutic use. They developed EHop-016 (Figure 3a), which blocks the interaction of the RAC-GEF Vav2 with a nucleotide-free RAC1 (G15A), which has a 100-fold higher affinity for activated GEFs, without affecting the association of the RAC-GEF TIAM-1 with RAC1 at micro-molar concentrations. EHop-016 decreases RAC downstream effects of PAK1 (p21-activated kinase 1) activity and directed migration of metastatic cancer cells(54). EHop-016 and other compounds under development may hold potential as a targeted therapeutics for the treatment of metastatic cancers with high RAC1 activity.
RAC1 inhibition for drug-resistant tumors
Acquired resistance to targeted therapeutics remains a fundamental cause of relapse and failure. The treatment of resistant tumors has proved a major challenge to the field of cancer therapeutics. Efforts to elucidate mechanisms of resistance continue to identify targets that might prove useful in overcoming resistance. RAC1 and its closely-related partners continue to emerge as critical mediators of resistance in diverse situations.
Perhaps the most well-characterized model for RAC-mediated resistance is that described in HER-2 positive breast tumors. RAC1 expression increases with inactivation of PTEN and with overexpression of IGF-1R(55), two mechanisms that underlie acquired resistance to treatment with anti-HER2/neu therapies. Reducing the activity of this pathway using either the small molecule RAC1 inhibitor NSC23766 or by siRNA knockdown of TIAM1 resensitizes trastuzumab-resistant breast tumors to that same agent by preventing the endocytic down-regulation of HER2 receptors[44]. High-throughput screens using RNAi to targeting genes commonly amplified in breast tumors with acquired resistance to HER2-targeted therapies identified RAC1 amplification as one of the most biologically relevant mechanisms of resistance. The subsequent inhibition of RAC1 restored sensitivity to lapatinib-resistant tumors(56).
Other studies remain underway to explore the ability of RAC1 inhibition to resensitize resistant ovarian cancers and leukemic cells to chemotherapy (cisplatin, methotrexate), with promising initial results presented at recent meetings(57). With the study of resistance mechanisms maturing and expanding, it will be interesting to see the extent of involvement that RAC has in mediating resistance to both targeted therapy and to traditional chemotherapy.
Targeting other members of the RAC pathway
Some studies indicate that greater specificity might be achieved by targeting the downstream effectors of RAC activity. One important effector of RAC activity is p67phox, which combines with other components of the NADPH oxidase system when activated to generate a fully functional complex for producing reactive oxygen species (ROS). This complex has been a target for investigators who aim to moderate the consequences of inflammation. Bosco et al have shown proof of principle in targeting this pathway with the development of Phox-I1 (Figure 3b), which targets a pocket formed when RAC1 complexes with p67phox(58). Phox-I1 and several derivative compounds seem to be effective at inhibiting the RAC-dependent generation of reactive oxygen species in a number of in vitro models of inflammation.
Other downstream targets are classical kinases and more readily targeted with traditional pharmacologic approaches. Bristol-Myers Squibb has developed a series of compounds that inhibit LIM kinases(59). Several of the compounds designed to target the LIM kinases, however, have had cytotoxic off-target effects on microtubule formation, while a few do not (such as compound 3, Figure 3b). These less-cytotoxic compounds appear in early studies to block some of the invasive phenotypes seen in breast cancer and squamous cell carcinoma cell lines(60).
Much recent effort has also gone into the development of different types of PAK inhibitors. One small molecule inhibitor, OSU 03012 (Figure 3b), is a derivative of the cyclooxygenase inhibitor celecoxib which was originally developed to target PDK1(61), but was subsequently shown to inhibit PAK1 activity at even lower concentrations (within the 1μM range(62)). This compound also appears to inhibit JAK/STAT and MAPK pathways as well. Limited studies with this compound in preclinical in vitro tumor models have shown that it inhibits cell proliferation in thyroid cancer cell lines and decreases the motility of those same cells(62). This compound also reverses resistance to imatinib mesylate in some cell lines(63) and sensitizes transformed astrocytes to killing by both radiation and by PI3K/AKT or MAPKK1/2 inhibitors. In small studies, it has been shown to restore tamoxifen sensitivity in xenograft models of breast cancer(64).
IPA-3 has been developed as a non-ATP-competitive PAK1 inhibitor(65) (Figure 3b). It is highly selective and potent (IC50 of about 2.5 μM), but has shown some chemical characteristics, such as covalent binding to the target, that make it less appealing for in vivo or therapeutic use. Higher concentrations of IPA-3 are necessary to inhibit PAKs as its activity requires a large transferable pool of the compound in the tumor microenvironment. A few small studies have published preclinical data showing that IPA-3 can induce apoptosis in a number of cancer cell lines(66), decrease cell spreading and adhesion in Scwannoma cell lines(67), and overcome resistance to phosphatidylinositol 3-kinase pathway inhibitors in some lymphomas(65). This same drug will block the transformation of breast tumor cells to form a multi-acinar phenotype in 3D culture(68).
An organometallic compound, which uses a bulky ruthenium core to create a selective allosteric PAK1 inhibitor, was recently reported(69). This compound, Λ-OS2 (Figure 3b), shows high selectivity for PAK1 and an IC50 around 350 nM(70). Several studies have been reported using similar compounds targeting other kinases, but further characterization of the effects of Λ-OS2 in living systems remains forthcoming.
RAC1 and the therapeutic window
Studies performed using inhibitors of RAC1 in vivo are limited, making inferences as to the likely therapeutic window speculative, but worth discussion. We can begin to make some educated guesses as to what toxicities might occur based on observations made in knockout mice. While RAC1 knockout is embryonic lethal, causing defects in germ layer formation, a number of tissue-specific RAC1 knockouts have been viable(71). Many phenotypes observed in these mice resulted from effects on embryonic development and would not likely be of consequence in mature organisms. Other phenotypes offer insights into the potential adverse effects of treatment with RAC1 inhibitors. These include: hair loss, cardiac hypertrophy, ineffective immune cell chemotaxis, and impaired neoangiogenesis. One should be careful in assuming a likely toxicity from such studies, however, since 1) gene knockout does not necessarily equate with pharmacologic inhibition and 2) the effects of inhibition on different RAC functions is likely dose-dependent. RAC performs functions both as a scaffolding protein and as a specific regulator of downstream targets. Thus, loss of the entire protein through genetic deletion may have quite a different effect from that of inhibiting a particular function.
Other inferences may be made from described roles of RAC1 in normal physiology. Given the well-described role for RAC1 in insulin sensitivity and glucose uptake in skeletal muscle(72), one might speculate that RAC inhibition would induce a state of relative insulin resistance and prevent contraction-related glucose uptake in skeletal muscle(73). It will be interesting to see which of these effects have true biological relevance and which will be of no specific consequence when investigators begin to report in vivo studies.
Conclusions
RAC1 has recently emerged as a critical regulator of tumor angiogenesis and metastasis and a promising therapeutic target for cancer drug discovery. From a mechanistic perspective, several important questions require further elucidation e.g the mechanisms underlying the reduced tumor vascular perfusion induced by RAC1 inhibition remain unclear. Collective studies serve to illustrate the pivotal role that RAC1 plays in the development and progression of diverse tumor types. The data are especially strong with respect to RAC1’s role in angiogenic and invasive behaviors. Targeting RAC1 and its associated pathways is an interesting strategy that could have marked effects on these malignant behaviors. Interestingly, such strategies may also help to reverse certain mechanisms of resistance to other targeted therapeutics, such as aberrant down-regulation of surface receptor expression in some models and the aberrant overexpression of hormone receptors in others. Few of the current pharmacologic candidates show properties that would make them acceptable for clinical use, but they have been improving with each iteration, both in potency and specificity. The preclinical data to date consistently supports the idea that modulating these pathways may have very desirable effects on tumor progression and continues to warrant ongoing development of newer and more specific agents. From a therapeutic perspective, it will be instrumental to classify the tumor types that will benefit most from anti-RAC1 therapy and to further validate combination approaches with existing anti-angiogenic and chemotherapeutic regimens. As with any biological system, effective manipulation of the RAC1 network to affect the desired outcomes may not be simply a matter of modulating overall RAC activity. This is illustrated by the studies of D4-GDI, in which lifting this RAC-1 inhibition yields the seemingly paradoxical effect of blocking tumor growth and spread. The manipulation of individual RAC-mediated processes through the inhibition of different members of its regulatory network may have a divergence of consequences. Further studies into the precise function of RAC1 in the tumor microenvironment, both on the cancer cells themselves as well as on the surrounding stromal and endothelial cells, should help us to understand the consequences of modulating these pathways. These studies will also provide further insights to fuel novel approaches for combating these malignancy-associated cellular processes.
Acknowledgments
Support: HK Bid is a recipient of a Pelotonia Fellowship from The Ohio State University. This review was supported in part by PHS award CA165995 to PJ Houghton.
Footnotes
Disclosure: The authors consider that there are no issues they believe could be construed as resulting in an actual, potential, or perceived conflict of interest with regard to the manuscript submitted.
References
- 1.Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays. 2007;29:356–70. doi: 10.1002/bies.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 3.Saci A, Cantley Lewis C, Carpenter Christopher L. Rac1 Regulates the Activity of mTORC1 and mTORC2 and Controls Cellular Size. Molecular Cell. 2011;42:50–61. doi: 10.1016/j.molcel.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehrlich JS, Hansen MDH, Nelson WJ. Spatio-Temporal Regulation of Rac1 Localization and Lamellipodia Dynamics during Epithelial Cell-Cell Adhesion. Dev Cell. 2002;3:259–70. doi: 10.1016/s1534-5807(02)00216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosco EE, Nakai Y, Hennigan RF, Ratner N, Zheng Y. NF2-deficient cells depend on the Rac1-canonical Wnt signaling pathway to promote the loss of contact inhibition of proliferation. Oncogene. 2010;29:2540–9. doi: 10.1038/onc.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rathinam R, Berrier A, Alahari SK. Role of Rho GTPases and their regulators in cancer progression. Frontiers in bioscience : a journal and virtual library. 2011;16:2561–71. doi: 10.2741/3872. [DOI] [PubMed] [Google Scholar]
- 7.Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–9. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Eden S, Rohatgi R, Podtelejnikov AV, Mann M, Kirschner MW. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature. 2002;418:790–3. doi: 10.1038/nature00859. [DOI] [PubMed] [Google Scholar]
- 9.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348(Pt 2):241–55. [PMC free article] [PubMed] [Google Scholar]
- 10.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–80. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 11.Watt FM, Frye M, Benitah SA. MYC in mammalian epidermis: how can an oncogene stimulate differentiation? Nature Reviews Cancer. 2008;8:234–42. doi: 10.1038/nrc2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 13.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan W, Palmby TR, Gavard J, Amornphimoltham P, Zheng Y, Gutkind JS. An essential role for Rac1 in endothelial cell function and vascular development. FASEB J. 2008;22:1829–38. doi: 10.1096/fj.07-096438. [DOI] [PubMed] [Google Scholar]
- 15.Liu H, Rigamonti D, Badr A, Zhang J. Ccm1 regulates microvascular morphogenesis during angiogenesis. J Vasc Res. 2011;48:130–40. doi: 10.1159/000316851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koh W, Mahan RD, Davis GE. Cdc42- and Rac1-mediated endothelial lumen formation requires Pak2, Pak4 and Par3, and PKC-dependent signaling. J Cell Sci. 2008;121:989–1001. doi: 10.1242/jcs.020693. [DOI] [PubMed] [Google Scholar]
- 17.D’Amico G, Jones DT, Nye E, Sapienza K, Ramjuan AR, Reynolds LE, et al. Regulation of lymphatic-blood vessel separation by endothelial Rac1. Development. 2009;136:4043–53. doi: 10.1242/dev.035014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell–cell adhesion. J Cell Biol. 2007;178:517–27. doi: 10.1083/jcb.200701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connolly JO, Simpson N, Hewlett L, Hall A. Rac regulates endothelial morphogenesis and capillary assembly. Mol Biol Cell. 2002;13:2474–85. doi: 10.1091/mbc.E02-01-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vader P, van der Meel R, Symons M, Fens M, Pieters E, Wilschut K, et al. Examining the role of Rac1 in tumor angiogenesis and growth: a clinically relevant RNAi-mediated approach. Angiogenesis. 2011;14:457–66. doi: 10.1007/s10456-011-9229-x. [DOI] [PubMed] [Google Scholar]
- 21.Brantley-Sieders DM, Zhuang G, Vaught D, Freeman T, Hwang Y, Hicks D, et al. Host-deficiency in Vav 2/3 guanine nucleotide exchange factors impairs tumor growth, survival, and angiogenesis in vivo. Mol Cancer Res. 2009;7:615–23. doi: 10.1158/1541-7786.MCR-08-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Amico G, Robinson SD, Germain M, Reynolds LE, Thomas GJ, Elia G, et al. Endothelial-Rac1 is not required for tumor angiogenesis unless alphavbeta3-integrin is absent. PLoS One. 2010;5:e9766. doi: 10.1371/journal.pone.0009766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 25.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9:285–93. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parri M, Chiarugi P. Rac and Rho GTPases in cancer cell motility control. Cell Commun Signal. 2010:8. doi: 10.1186/1478-811X-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baugher PJ, Krishnamoorthy L, Price JE, Dharmawardhane SF. Rac1 and Rac3 isoform activation is involved in the invasive and metastatic phenotype of human breast cancer cells. Breast Cancer Res. 2005;7:R965–74. doi: 10.1186/bcr1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamai T, Shirataki H, Nakanishi K, Furuya N, Kambara T, Abe H, et al. Increased Rac1 activity and Pak1 overexpression are associated with lymphovascular invasion and lymph node metastasis of upper urinary tract cancer. BMC Cancer. 2010;10:164. doi: 10.1186/1471-2407-10-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel V, Rosenfeldt HM, Lyons R, Servitja J-M, Bustelo XR, Siroff M, et al. Persistent activation of Rac1 in squamous carcinomas of the head and neck: evidence for an EGFR/Vav2 signaling axis involved in cell invasion. Carcinogenesis. 2007;28:1145–52. doi: 10.1093/carcin/bgm008. [DOI] [PubMed] [Google Scholar]
- 30.Chan AY, Coniglio SJ, Chuang Y-y, Michaelson D, Knaus UG, Philips MR, et al. Roles of the Rac1 and Rac3 GTPases in human tumor cell invasion. Oncogene. 2005;24:7821–9. doi: 10.1038/sj.onc.1208909. [DOI] [PubMed] [Google Scholar]
- 31.Chang CY, Lin SC, Su WH, Ho CM, Jou YS. Somatic LMCD1 mutations promoted cell migration and tumor metastasis in hepatocellular carcinoma. Oncogene. 2012;31:2640–52. doi: 10.1038/onc.2011.440. [DOI] [PubMed] [Google Scholar]
- 32.Wu L, Cai C, Wang X, Liu M, Li X, Tang H. MicroRNA-142-3p, a new regulator of RAC1, suppresses the migration and invasion of hepatocellular carcinoma cells. FEBS Lett. 2011;585:1322–30. doi: 10.1016/j.febslet.2011.03.067. [DOI] [PubMed] [Google Scholar]
- 33.Gramantieri L, Ferracin M, Fornari F, Veronese A, Sabbioni S, Liu CG, et al. Cyclin G1 is a target of miR-122a, a microRNA frequently down-regulated in human hepatocellular carcinoma. Cancer Res. 2007;67:6092–9. doi: 10.1158/0008-5472.CAN-06-4607. [DOI] [PubMed] [Google Scholar]
- 34.Qin J, Xie Y, Wang B, Hoshino M, Wolff DW, Zhao J, et al. Upregulation of PIP3-Dependent Rac Exchanger 1 (P-Rex1) Promotes Prostate Cancer Metastasis. Oncogene. 2009;28:1853–63. doi: 10.1038/onc.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, et al. Rac Activation and Inactivation Control Plasticity of Tumor Cell Movement. Cell. 2008;135:510–23. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 36.Mula RV, Bhatia V, Falzon M. PTHrP promotes colon cancer cell migration and invasion in an integrin alpha6beta4-dependent manner through activation of Rac1. Cancer Lett. 2010;298:119–27. doi: 10.1016/j.canlet.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauer NN, Chen YW, Samant RS, Shevde LA, Fodstad O. Rac1 activity regulates proliferation of aggressive metastatic melanoma. Exp Cell Res. 2007;313:3832–9. doi: 10.1016/j.yexcr.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 38.Matos P, Jordan P. Increased Rac1b expression sustains colorectal tumor cell survival. Mol Cancer Res. 2008;6:1178–84. doi: 10.1158/1541-7786.MCR-08-0008. [DOI] [PubMed] [Google Scholar]
- 39.Wang SE, Yu Y, Criswell TL, Debusk LM, Lin PC, Zent R, et al. Oncogenic mutations regulate tumor microenvironment through induction of growth factors and angiogenic mediators. Oncogene. 2010;29:3335–48. doi: 10.1038/onc.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mossessova E, Corpina RA, Goldberg J. Crystal structure of ARF1*Sec7 complexed with Brefeldin A and its implications for the guanine nucleotide exchange mechanism. Mol Cell. 2003;12:1403–11. doi: 10.1016/s1097-2765(03)00475-1. [DOI] [PubMed] [Google Scholar]
- 41.Renault L, Guibert B, Cherfils J. Structural snapshots of the mechanism and inhibition of a guanine nucleotide exchange factor. Nature. 2003;426:525–30. doi: 10.1038/nature02197. [DOI] [PubMed] [Google Scholar]
- 42.Vigil D, Cherfils J, Rossman KL, Der CJ. Ras superfamily GEFs and GAPs: validated and tractable targets for cancer therapy? Nature Reviews Cancer. 2010;10:842–57. doi: 10.1038/nrc2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci U S A. 2004;101:7618–23. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gastonguay A, Berg T, Hauser AD, Schuld N, Lorimer E, Williams CL. The role of Rac1 in the regulation of NF-kappaB activity, cell proliferation, and cell migration in non-small cell lung carcinoma. Cancer Biol Ther. 2012;13:647–56. doi: 10.4161/cbt.20082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dokmanovic M, Hirsch DS, Shen Y, Wu WJ. Rac1 contributes to trastuzumab resistance of breast cancer cells: Rac1 as a potential therapeutic target for the treatment of trastuzumab-resistant breast cancer. Mol Cancer Ther. 2009;8:1557–69. doi: 10.1158/1535-7163.MCT-09-0140. [DOI] [PubMed] [Google Scholar]
- 46.Moran EM, Connolly M, Gao W, McCormick J, Fearon U, Veale DJ. Interleukin-17A induction of angiogenesis, cell migration, and cytoskeletal rearrangement. Arthritis Rheum. 2011;63:3263–73. doi: 10.1002/art.30582. [DOI] [PubMed] [Google Scholar]
- 47.Sawada N, Salomone S, Kim HH, Kwiatkowski DJ, Liao JK. Regulation of endothelial nitric oxide synthase and postnatal angiogenesis by Rac1. Circ Res. 2008;103:360–8. doi: 10.1161/CIRCRESAHA.108.178897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colomba A, Giuriato S, Dejean E, Thornber K, Delsol G, Tronchere H, et al. Inhibition of Rac controls NPM-ALK-dependent lymphoma development and dissemination. Blood Cancer J. 2011;1:e21. doi: 10.1038/bcj.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomas EK, Cancelas JA, Chae HD, Cox AD, Keller PJ, Perrotti D, et al. Rac guanosine triphosphatases represent integrating molecular therapeutic targets for BCR-ABL-induced myeloproliferative disease. Cancer Cell. 2007;12:467–78. doi: 10.1016/j.ccr.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida T, Zhang Y, Rivera Rosado LA, Chen J, Khan T, Moon SY, et al. Blockade of Rac1 activity induces G1 cell cycle arrest or apoptosis in breast cancer cells through downregulation of cyclin D1, survivin, and X-linked inhibitor of apoptosis protein. Mol Cancer Ther. 2010;9:1657–68. doi: 10.1158/1535-7163.MCT-09-0906. [DOI] [PubMed] [Google Scholar]
- 51.Shutes A, Onesto C, Picard V, Leblond B, Schweighoffer F, Der CJ. Specificity and mechanism of action of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. J Biol Chem. 2007;282:35666–78. doi: 10.1074/jbc.M703571200. [DOI] [PubMed] [Google Scholar]
- 52.Rosenblatt AE, Garcia MI, Lyons L, Xie Y, Maiorino C, Desire L, et al. Inhibition of the Rho GTPase, Rac1, decreases estrogen receptor levels and is a novel therapeutic strategy in breast cancer. Endocrine-related cancer. 2011;18:207–19. doi: 10.1677/ERC-10-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferri N, Corsini A, Bottino P, Clerici F, Contini A. Virtual screening approach for the identification of new Rac1 inhibitors. Journal of medicinal chemistry. 2009;52:4087–90. doi: 10.1021/jm8015987. [DOI] [PubMed] [Google Scholar]
- 54.Montalvo-Ortiz BL, Castillo-Pichardo L, Hernandez E, Humphries-Bickley T, De la Mota-Peynado A, Cubano LA, et al. Characterization of EHop-016, novel small molecule inhibitor of Rac GTPase. J Biol Chem. 2012;287:13228–38. doi: 10.1074/jbc.M111.334524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao Y, Wang Z, Jiang Y, Yang C. Inactivation of Rac1 reduces Trastuzumab resistance in PTEN deficient and insulin-like growth factor I receptor overexpressing human breast cancer SKBR3 cells. Cancer Lett. 2011;313:54–63. doi: 10.1016/j.canlet.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 56.Wetterskog D, Shiu KK, Chong I, Meijer T, Mackay A, Lambros M, et al. Identification of novel determinants of resistance to lapatinib in ERBB2-amplified cancers. Oncogene. 2013 doi: 10.1038/onc.2013.41. [DOI] [PubMed] [Google Scholar]
- 57.Goncharov K, Zeineldin R. Sensitizing resistant ovarian cancer to chemotherapy through inhibition of small GTPases. Cancer Res. 2012;72(8 Suppl):Abstract nr 4227. [Google Scholar]
- 58.Bosco EE, Kumar S, Marchioni F, Biesiada J, Kordos M, Szczur K, et al. Rational design of small molecule inhibitors targeting the Rac GTPase-p67(phox) signaling axis in inflammation. Chemistry & biology. 2012;19:228–42. doi: 10.1016/j.chembiol.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ross-Macdonald P, de Silva H, Guo Q, Xiao H, Hung CY, Penhallow B, et al. Identification of a nonkinase target mediating cytotoxicity of novel kinase inhibitors. Mol Cancer Ther. 2008;7:3490–8. doi: 10.1158/1535-7163.MCT-08-0826. [DOI] [PubMed] [Google Scholar]
- 60.Scott RW, Hooper S, Crighton D, Li A, Konig I, Munro J, et al. LIM kinases are required for invasive path generation by tumor and tumor-associated stromal cells. J Cell Biol. 2010;191:169–85. doi: 10.1083/jcb.201002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu J, Huang JW, Tseng PH, Yang YT, Fowble J, Shiau CW, et al. From the cyclooxygenase-2 inhibitor celecoxib to a novel class of 3-phosphoinositide-dependent protein kinase-1 inhibitors. Cancer Res. 2004;64:4309–18. doi: 10.1158/0008-5472.CAN-03-4063. [DOI] [PubMed] [Google Scholar]
- 62.Porchia LM, Guerra M, Wang YC, Zhang Y, Espinosa AV, Shinohara M, et al. 2-amino-N-{4-[5-(2-phenanthrenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]-phenyl} acetamide (OSU-03012), a celecoxib derivative, directly targets p21-activated kinase. Molecular pharmacology. 2007;72:1124–31. doi: 10.1124/mol.107.037556. [DOI] [PubMed] [Google Scholar]
- 63.Tseng PH, Lin HP, Zhu J, Chen KF, Hade EM, Young DC, et al. Synergistic interactions between imatinib mesylate and the novel phosphoinositide-dependent kinase-1 inhibitor OSU-03012 in overcoming imatinib mesylate resistance. Blood. 2005;105:4021–7. doi: 10.1182/blood-2004-07-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weng SC, Kashida Y, Kulp SK, Wang D, Brueggemeier RW, Shapiro CL, et al. Sensitizing estrogen receptor-negative breast cancer cells to tamoxifen with OSU-03012, a novel celecoxib-derived phosphoinositide-dependent protein kinase-1/Akt signaling inhibitor. Mol Cancer Ther. 2008;7:800–8. doi: 10.1158/1535-7163.MCT-07-0434. [DOI] [PubMed] [Google Scholar]
- 65.Deacon SW, Beeser A, Fukui JA, Rennefahrt UE, Myers C, Chernoff J, et al. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chemistry & biology. 2008;15:322–31. doi: 10.1016/j.chembiol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ong CC, Jubb AM, Haverty PM, Zhou W, Tran V, Truong T, et al. Targeting p21-activated kinase 1 (PAK1) to induce apoptosis of tumor cells. Proc Natl Acad Sci U S A. 2011;108:7177–82. doi: 10.1073/pnas.1103350108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Flaiz C, Chernoff J, Ammoun S, Peterson JR, Hanemann CO. PAK kinase regulates Rac GTPase and is a potential target in human schwannomas. Experimental neurology. 2009;218:137–44. doi: 10.1016/j.expneurol.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arias-Romero LE, Villamar-Cruz O, Pacheco A, Kosoff R, Huang M, Muthuswamy SK, et al. A Rac-Pak signaling pathway is essential for ErbB2-mediated transformation of human breast epithelial cancer cells. Oncogene. 2010;29:5839–49. doi: 10.1038/onc.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feng L, Geisselbrecht Y, Blanck S, Wilbuer A, Atilla-Gokcumen GE, Filippakopoulos P, et al. Structurally sophisticated octahedral metal complexes as highly selective protein kinase inhibitors. Journal of the American Chemical Society. 2011;133:5976–86. doi: 10.1021/ja1112996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang S, Suvannasankha A, Crean CD, White VL, Johnson A, Chen CS, et al. OSU-03012, a novel celecoxib derivative, is cytotoxic to myeloma cells and acts through multiple mechanisms. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:4750–8. doi: 10.1158/1078-0432.CCR-07-0136. [DOI] [PubMed] [Google Scholar]
- 71.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nature reviews Molecular cell biology. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 72.Chiu TT, Jensen TE, Sylow L, Richter EA, Klip A. Rac1 signalling towards GLUT4/glucose uptake in skeletal muscle. Cellular signalling. 2011;23:1546–54. doi: 10.1016/j.cellsig.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 73.Sylow L, Jensen TE, Kleinert M, Mouatt JR, Maarbjerg SJ, Jeppesen J, et al. Rac1 is a novel regulator of contraction-stimulated glucose uptake in skeletal muscle. Diabetes. 2013;62:1139–51. doi: 10.2337/db12-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]