Abstract
Estradiol affects hippocampal-dependent spatial memory and underlying structural and electrical synaptic plasticity in female mice and rats. Using estrogen receptor (ER) alpha and beta knockout mice and wild-type littermates, we investigated the role of ERs in estradiol effects on multiple pathways important for hippocampal plasticity and learning. Six hours of estradiol administration increased immunoreactivity for phosphorylated Akt throughout the hippocampal formation, while 48 hours of estradiol increased immunoreactivity for phosphorylated TrkB receptor. Estradiol effects on phosphorylated Akt and TrkB immunoreactivities were abolished in ER alpha and ER beta knockout mice. Estradiol also had distinct effects on immunoreactivity for PSD-95 and BDNF mRNA in ER alpha and beta knockout mice. Thus, estradiol acts through both ERs alpha and beta in several subregions of the hippocampal formation. The different effects of estradiol at 6 and 48 hours indicate that several mechanisms of estrogen receptor signaling contribute to this female hormone’s influence on hippocampal synaptic plasticity. By further delineating these mechanisms, we will better understand and predict the effects of endogenous and exogenous ovarian steroids on mood, cognition, and other hippocampal-dependent behaviors.
Keywords: estrogen receptor, estrogen, synaptic plasticity, hippocampus
Introduction
Growing evidence indicates that plasticity of individual synapses underlies learning and behavior modification. This synaptic plasticity comprises experience-dependent changes in synaptic structure, including the density, size, and shape of spine synapses, as well as changes in the efficacy or magnitude of synaptic potentiation (Engert and Bonhoeffer, 1999, Muller et al., 2000, Whitlock et al., 2006). Factors that influence any one of these types of synaptic plasticity may alter animal behavior (Leuner et al., 2003, Whitlock et al., 2006, Hongpaisan and Alkon, 2007, Costa-Mattioli et al., 2009).
The ovarian steroid hormone, estradiol, enhances several measures of synaptic plasticity in the rodent hippocampal formation. Estradiol increases the size and density of hippocampal dendritic spines in the CA1 region of female rats and mice (Gould et al., 1990, Woolley et al., 1990, Woolley and McEwen, 1992, Murphy and Segal, 1996, Li et al., 2004, Gonzalez-Burgos et al., 2005). Estradiol also enhances hippocampal excitability and the magnitude of long-term potentiation in female rats (Warren et al., 1995, Cordoba Montoya and Carrer, 1997, Foy et al., 1999, Good et al., 1999, Scharfman et al., 2003, Kim et al., 2006, Scharfman and MacLusky, 2006, Smith and McMahon, 2006, Foy et al., 2008). The ability of this reproductive hormone to influence hippocampal synaptic plasticity may underlie its complex, task-dependent effects on hippocampal-dependent learning and memory (Singh et al., 1994, Daniel et al., 1997, Packard and Teather, 1997b, Warren and Juraska, 1997, Luine et al., 1998, Korol and Kolo, 2002, Luine et al., 2003, Korol et al., 2004, Li et al., 2004, Sandstrom and Williams, 2004, Gresack and Frick, 2006, Wallace et al., 2006, Xu and Zhang, 2006, Frye et al., 2007, Spencer et al., 2008b, Frick, 2009). It is likely that estradiol’s effects on hippocampal-dependent behavior are at least partially due to direct effects on that brain region, as estradiol enhances spatial memory when infused directly into the hippocampal formation of female mice and rats (Packard and Teather, 1997a, Zurkovsky et al., 2006, Fernandez et al., 2008, Fan et al., 2010).
Two types of classical estrogen receptors, alpha and beta, are expressed in the mammalian hippocampal formation (Shughrue et al., 1997, Weiland et al., 1997, Register et al., 1998, Mitra et al., 2003). These ERs both signal via nucleus-initiated signaling, the classical mode of steroid receptor signaling in which estrogen receptors activate new gene transcription by association with estrogen response elements (EREs) in the DNA (Nilsson et al., 2001). They can also signal via the more recently characterized membrane-initiated signaling, in which membrane-associated receptors cooperate with growth factor receptors or G protein-coupled receptors to activate kinase cascades (Levin, 2005, Hammes and Levin, 2007, Vasudevan and Pfaff, 2007). This membrane-initiated ER signaling occurs rapidly, with kinase signaling observable within minutes and up to six hours after hormone exposure (Akama and McEwen, 2003, Abraham et al., 2004, Lee et al., 2004, Fernandez et al., 2008, Yuen et al., 2011). In contrast, new gene products of nucleus-initiated signaling are first measurable 12–24 hours after hormone exposure (Vasudevan et al., 2001, Gottfried-Blackmore et al., 2007).
Both ERs alpha and beta are important for hippocampal function, as knockout of either receptor in mice impairs hippocampal-dependent learning (Fugger et al., 2000, Rissman et al., 2002, Day et al., 2005). Electron microscopy studies have revealed different distributions of ERs alpha and beta in hippocampal neurons, suggesting that they have different functions (McEwen and Milner, 2007). Both receptors have been implicated in synaptic potentiation, synaptic depression, and synapse formation in hippocampal neurons (Day et al., 2005, Szymczak et al., 2006, Liu et al.). But the distinct molecular pathways by which ERs alpha and beta affect hippocampal synaptic plasticity are unknown.
We recently showed that natural fluctuations in ovarian steroid hormones in female mice impact several molecules with well-known roles in hippocampal synaptic plasticity: the kinase Akt, the postsynaptic scaffolding protein PSD-95, and the brain-derived neurotrophic factor (BDNF) receptor, TrkB (Spencer et al., 2008a). PI3 kinase/Akt signaling is involved in a wide array of neuronal functions, including cell survival and long-term potentiation (Sanna et al., 2002, Sui et al., 2008, Yang et al., 2008), while PSD-95 is essential for the activity-driven formation and stabilization of excitatory spine synapses (El-Husseini et al., 2000, Ehrlich et al., 2007). BDNF supports several aspects of synaptic plasticity, including protein-synthesis-dependent long-term potentiation (LTP) and spine structure modification, through binding to its receptor TrkB (Kang and Schuman, 1995, Messaoudi et al., 2002, Bekinschtein et al., 2008, Tanaka et al., 2008). Levels of phosphorylated Akt, PSD-95 protein, BDNF mRNA, and phosphorylated TrkB increase during proestrus, the high-estradiol stage of the mouse estrous cycle (Spencer et al., 2008a, Spencer et al., 2010). The influence of estradiol on these endpoints could account for natural fluctuations in synaptic plasticity and behavior across the estrous cycle (Frick and Berger-Sweeney, 2001, Scharfman et al., 2003, Spencer et al., 2010).
To better understand how estradiol influences these important molecular targets, we investigated the effects of controlled estradiol treatment in wild-type ovariectomized mice on the phosphorylation or expression of Akt, PSD-95, BDNF, and TrkB in the dorsal hippocampal formation. The dorsal hippocampal formation is the region where effects of estradiol have previously been demonstrated on spine and synapse density, synaptic protein expression, and Akt and BDNF signaling (e.g. (Li et al., 2004, Spencer et al., 2008a); for review, see (Spencer et al., 2008b)). The mice were treated with estradiol benzoate or oil vehicle for 6 or 48 hours. The 6-hour time point was chosen based on previous studies showing that estradiol increases Akt phosphorylation in neuronal cell lines after 30 minutes up to at least 6 hours of exposure (Akama and McEwen, 2003). The 48-hour time point was chosen based on previous findings that 48 hours of estradiol treatment increases BDNF expression in the rat hippocampus (Gibbs, 1998). As indicated above, these two time points are consistent with relatively rapid, membrane-initiated actions (at 6 hours), and slower, nucleus-initiated events requiring new gene transcription (at 48 hours). Immunoreactivity for phosphorylated Akt, phosphorylated TrkB, PSD-95, BDNF mRNA, and TrkB mRNA was measured using the same techniques of quantitative immunocytochemistry and in situ hybridization as in our previous studies (Spencer et al., 2008a, Spencer et al., 2010). This consistency facilitated the comparison of our results between studies, and also allowed for anatomic resolution of estradiol effects in different subregions of the hippocampal formation.
Based on the results in wild-type mice, we then utilized knockout mice missing ER alpha (AERKO) or beta (BERKO) to further examine the role of estrogen receptors in estradiol actions in the dorsal hippocampal formation.
Methods
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee of The Rockefeller University and the University of Tsukuba and were in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Mice were housed at the University of Tsukuba on a 12-hour light/dark cycle with food and water available ad libitum for the duration of the studies. Mice were adult females between the ages of 9 and 20 weeks, with ages counterbalanced across groups.
AERKO and BERKO mice were previously generated by targeted disruption of the ER alpha and beta genes respectively and bred onto a C57Bl/6J background (Lubahn et al., 1993, Krege et al., 1998). They were back-crossed for at least ten generations before they were received in our laboratory. All pups obtained from mating of heterozygous mice in each colony were genotyped as previously described (Lubahn et al., 1993, Krege et al., 1998). Homozygous knockout mice and wild-type littermates were used for all experiments.
Ovariectomy and estrogen replacement
Mice (38 WT, 23 AERKO, and 25 BERKO) were ovariectomized under isoflurane anesthesia and recovered from surgery for one week. Subcutaneous injections of 5 µg estradiol benzoate in 100 µl vehicle (sesame oil) or vehicle alone were then delivered. For the 6-hour treatment, one injection was delivered six hours before the mice were killed. For the 48-hour treatment, two injections were given 24 hours apart, and the mice were killed 24 hours after the second injection, 48 hours after the start of treatment. In pilot experiments, this 48-hour treatment paradigm resulted in average serum estradiol levels of 51 +/− 6 pg/mL, similar to the proestrus peak in serum estradiol levels (Walmer et al., 1992, Spencer et al., 2008a).
Tissue Collection
Mice were anesthetized with an overdose of sodium pentobarbital (150 mg/kg intraperitoneal) and perfused through the aorta with saline/heparin followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4; PB). Brains were removed from the skull and postfixed in 4% paraformaldehyde for 1 hour. They were then sunk in 30% sucrose in PB for 48 hours at 4°C, frozen at −80°, and shipped on dry ice via Federal Express to The Rockefeller University in New York City. Sections (40 µm thick) through the hippocampal formation were cut on a freezing microtome (Microm HM440E) into PB. Sections were stored at 20°C in a cryoprotectant solution of 50% ethylene glycol, 15% sucrose in phosphate-buffered saline (PBS) until use.
Tissue Selection
Sections through the dorsal hippocampal formation for in situ hybridization and immunocytochemistry were selected from level 29 of Paxinos and Watson, bregma 0.26 mm (Paxinos and Watson, 1998) as previously described (Znamensky et al., 2003). For each endpoint, sections were matched to ensure that they were from the same level for each animal. Several steps were taken to insure identical labeling conditions (Pierce et al., 1999). Tissue from each experimental condition was marked with identifying punches so that all conditions were pooled on each slide (for in situ hybridization) or into individual crucibles (for immunocytochemistry). Tissue from the 6- and 48-hour treatment groups was processed separately, but within a time point, all slides/crucibles were processed together for each endpoint.
In situ hybridization
Antisense oligonucleotides matching the BDNF exon IX (5'GGG TTA CAC GAA GGA AGG CTG CAG GGG CAT AGA CAA AAG GCA CTG GAA CT3') and TrkB (5' TGC GAC TGC GTC AGC TCG GTG GGC GGG TTC CCT CTG CCA TCA GCA CTG C 3') sequences were obtained from Integrated DNA Technologies (Coralville, IA). Control sense sequences were also generated and used as a control to confirm a low level of nonspecific interactions. Floating sections containing dorsal hippocampus were mounted on Fisher Superfrost Plus slides, washed in 0.05 M PB, and dried in a desiccator. The oligonucleotides were labeled with 33P an in situ hybridization conducted as previously described, starting after the formaldehyde fix step (Hunter et al., 2005). Briefly, sections underwent the following washes: deionized water, 0.1 M triethanolamine with 5ml/l acetic anhydride, salt–sodium citrate buffer (SSC), ascending concentrations of ethanol, chloroform, and 95% ethanol. Sections were then air dried and incubated for 2 h with hybridization buffer without probe, followed by one wash in 2X SSC, and one in 95% ethanol. Sections were air dried and incubated overnight at 42°C with labeled probe (0.5×106 counts per slide) diluted in 50% deionized formamide in hybridization buffer with dextran sulfate, denatured salmon testis DNA (Sigma, St. Louis, MO), and 200 mM dithiothreital. After hybridization, sections were washed 4 times in SSC at 55 °C and left in SSC to cool to room temperature. Final washes were as follows: 50% ethanol, 0.3 M ammonium acetate, 85% ethanol, 0.3 M ammonium acetate, and 100% ethanol. Slides were then air dried and exposed to Kodak MR autoradiography films.
Films were developed after a two-week exposure and images taken on a light box using a CoolSnap camera (Princeton Instruments, Trenton, NJ). Optical density of BDNF and TrkB was measured from the CA1 and CA3 pyramidal cell layers of the hippocampal formation and the granule cell layer of the dentate gyrus in both hemispheres and normalized to an area lacking labeling (CA1 stratum radiatum) using MCID image analysis software (Cambridge, England).
Antibodies
Serial dilution tests of each antibody established that the labeling intensity was linear, and antibody dilutions were chosen that produced slightly less than half-maximal labeling intensity to optimize the detection of intensity variations (Chang et al., 2000).
Monoclonal mouse anti-PSD-95 (1:20,000) was purchased from Sigma (St. Louis, MO). Specificity of this antibody was confirmed by Western Blot, where it recognized the same double band as an affinity-purified rabbit PSD-95 antiserum (Kornau et al., 1995).
Polyclonal rabbit anti-phosphothreonine 308 Akt (pakt; 1:1500) was purchased from Cell Signaling Technology (Danvers, MA). Specificity of the antibody was confirmed by Western blot, where it did not detect nonphosphorylated Akt, Akt phosphorylated at other sites, or other members of the same protein family (Akama and McEwen, 2003, Znamensky et al., 2003). In rats, the pattern of pAkt immunolabeling in the hippocampus obtained with the Cell Signaling antibody was identical to that obtained with anti-pAkt from Upstate Biotechnology (Znamensky et al., 2003).
Polyclonal rabbit anti-phosphotyrosine 816 TrkB (pTrkB; 1:1000) was a gift from Moses Chao at New York University. Western Blot, peptide pre-adsorption, and immunocytochemistry in TrkB heterozygous and knockout mice confirmed the specificity of this antibody (Arevalo et al., 2006, Bath et al., 2008, Spencer-Segal et al., 2011). By light microscopy, hippocampal pTrkB-ir is absent in TrkB knockout mice, and significantly diminished in TrkB heterozygous mice.
Immunocytochemistry and Densitometry
Sections were washed in 0.1 M Tris buffer, blocked in 0.5% bovine serum albumin (BSA) and incubated in primary antiserum to pAkt, PSD-95, or pTrkB for 24 hours at room temperature followed by 48 hours at 4°C. Sections were then incubated in: (1) biotinylated goat anti-rabbit or anti-mouse immunoglobulin (IgG) in 0.1% BSA (1:400; Vector Labs, Burlingame, CA) for 30 minutes, avidin biotin complex (ABC) for 30 minutes (Vector Labs, Burlingame, CA), and diaminobenzidine with H2O2 (Aldrich, Milwaukee, WI) for 6 minutes, with Tris washes in between each incubation. Sections then were mounted on Superfrost Plus slides (Thermo Fisher Scientific, Waltham, MA), air-dried, and coverslipped with DPX mounting medium (Aldrich, Milwaukee, WI).
Images for densitometry were taken on the same microscope, in the same session, with fixed settings. One brain section was analyzed per animal. The images were captured on a Nikon E800 microscope using a Dage CCD C72 camera and control unit using preset gain and black levels, a Data Translation Quick capture card, and NIH Image 1.50 software to a final magnification of 40x. To compensate for uneven illumination, blank fields from a slide without tissue were subtracted. To prepare figures, the levels, brightness and contrast were adjusted in Adobe Photoshop 7.0 on a Macintosh computer. Final figures were assembled in Microsoft Powerpoint, version X for Mac.
Quantitative Analysis was conducted using NIH Image as previously described (Spencer et al., 2008a, Torres-Reveron et al., 2009, Waters et al., 2009). Briefly, the average pixel density (of 256 gray levels) was determined for each of three selected regions: CA1 stratum radiatum, CA3 stratum radiatum, and the central hilus of the dentate gyrus (Fig. 1). For each region, three measurements were taken from different areas. To compensate for background staining, the average pixel density for 3 small regions that lack labeling in corpus callosum was determined within each captured image, and subtracted from all density measurements made on that image. For a given tissue section, the final optical density was the mean optical density from both hemispheres.
Fig. 1.
Micrograph shows areas sampled for densitometric analysis in a representative section through the dorsal hippocampal formation of a female wild-type mouse labeled with anti-pTrkB antibody. Three measurements were taken from each subregion including corpus callosum (CC), CA1 stratum radiatum (CA1 SR), CA3 stratum radiatum (CA3 SR), and hilus of the dentate gyrus (DG HIL). The average pixel density for CA1, CA3, and DG was corrected for background staining by subtracting the average pixel density for corpus callosum. Marker bar, 100 µm.
Quantitative and Statistical Analysis
Optical densities were compared for each endpoint, genotype, and time point using mixed-design two-way ANOVA with treatment as the between-subjects variable, and hippocampal subregion as the within-subjects variable. To determine whether levels of each endpoint were comparable between vehicle-treated AERKO and BERKO mice and wild-type littermates, optical densities were compared using two-way ANOVA with genotype as the between-subjects and hippocampal subregion as the within-subjects variable. Bonferroni post-hoc tests were performed whenever two-way ANOVA showed a significant main effect or interaction. No differences were observed between wild-type littermates of AERKO and BERKO mice for any endpoint, and so these were pooled for the final analysis. Significant differences were defined as a p value of <0.05.
Results
Estradiol treatment for 6 hours increases pAkt-ir via ERs alpha and beta
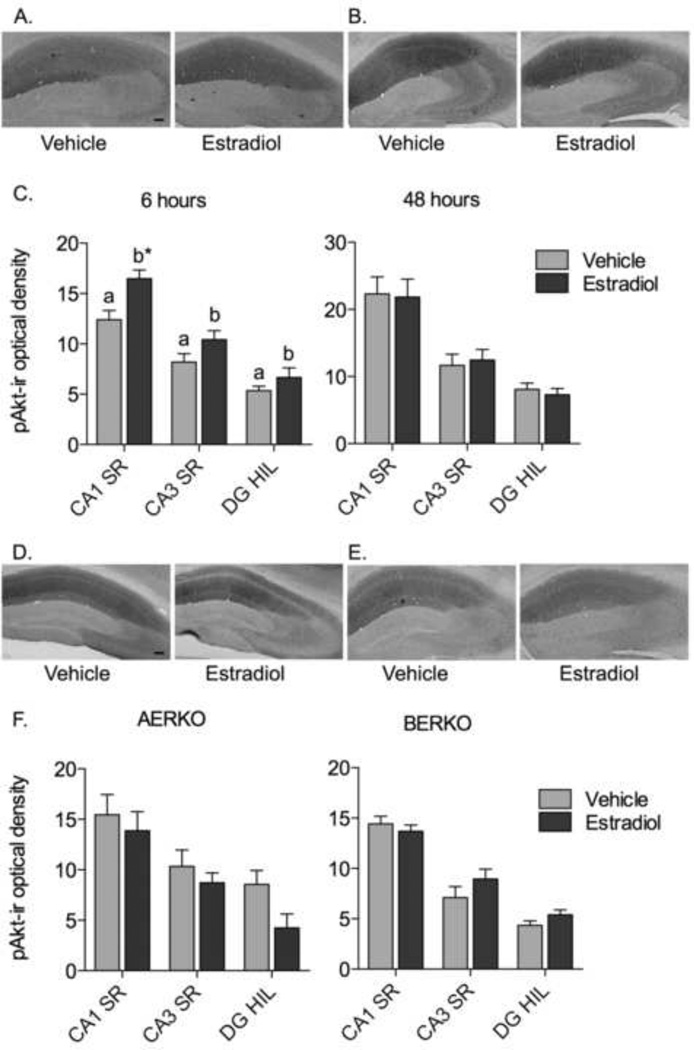
Ovariectomized wild-type AERKO and BERKO littermates (hereafter referred to as “wild-type mice”) were treated with 5 µg estradiol benzoate or oil vehicle for 6 or 48 hours, and pAkt-ir was assayed using immunocytochemistry and densitometry (Fig. 2). pAkt-ir was found in areas containing neuropil in the mouse hippocampal formation, with the densest labeling in the CA1 subregion (Fig. 2A-B). Two-way ANOVA showed a significant overall effect of hippocampal subregion for both 6- and 48-hour time points (F(2, 32) = 81, p < 0.0001 for 6 hours; F(2, 32) = 105, p < 0.0001 for 48 hours). Post-hoc tests showed that pAkt-ir was significantly higher in CA1 than in CA3 or dentate (p < 0.001 for both time points and treatment groups), and significantly higher in CA3 than in dentate (p < 0.01 for both time points and treatment groups). Estradiol treatment for 6 hours increased pAkt-ir relative to vehicle-treatment in all three hippocampal subregions (Fig. 2C); two-way ANOVA showed a significant overall effect of estradiol treatment at the 6-hour time point (F(1, 32) = 7.9, p = 0.013), with no interaction between treatment and subregion (F(2, 32) = 2.2, p = 0.12). Post-hoc tests showed a significant increase in pAkt-ir in CA1 stratum radiatum after estradiol treatment (p < 0.01). In contrast, after 48 hours of estradiol treatment, there was no difference in pAkt-ir between estradiol-treated mice and vehicle controls; two-way ANOVA showed no effect of treatment (F(1, 32) = 0.0054, p = 0.94), and no interaction between treatment and subregion (F(2, 32) = 0.35, p = 0.71).
Fig. 2.
Micrographs (A-B) show pAkt-ir in representative sections through the dorsal hippocampal formation of a female wild-type mouse treated with vehicle or estradiol for 6 hours (A) or 48 hours (B). Graphs (C) show the optical density of pAkt-ir after 6 and 48 hours of vehicle or estradiol treatment in wild-type mice. Micrographs (D-E) show immunocytochemistry for pAkt in representative sections through the dorsal hippocampal formation of female AERKO (D) and BERKO (E) mice treated with estradiol for 6 hours. Graphs (F) show the optical density of pAkt-ir after 6 hours of vehicle or estradiol treatment in AERKO or BERKO mice. Values from wild-type mice in AERKO and BERKO litters were pooled. Group “a” is significantly different from group “b,” p<0.05. * p < 0.05 relative to vehicle treatment. N = 9 for wild-type, 6 for AERKO (vehicle), 5 for AERKO (estradiol), 7 for BERKO. Marker bar, 100 µm.
To determine whether the increase in pAkt-ir after 6 hours of estradiol treatment requires ER alpha or beta, pAkt-ir was measured in AERKO and BERKO mice treated with estradiol or oil vehicle for 6 hours. pAkt-ir showed the same pattern in AERKO and BERKO as in wild-type mice (Fig. 2 D-E). As in the wild-type mice, pAkt-ir was densest in the CA1 region and lightest in the dentate hilus. Two-way ANOVA showed a significant effect of hippocampal subregion for both genotypes (Fig. 2F; F(2, 18) = 41, p <0.0001 for AERKO; F(2, 22) = 72, p <0.0001 for BERKO). Similar to wild-type mice, post-hoc tests showed that pAkt-ir was significantly higher in CA1 than in CA3 or dentate (p < 0.01 for both genotypes and treatment groups), and significantly higher in CA3 than in dentate (p < 0.05 for all except ERKO-vehicle group). In contrast to the wild-type mice, estradiol had no effect on pAkt-ir in AERKO or BERKO mice. Two-way ANOVA showed no effect of treatment or interaction between treatment and subregion for AERKO (F(1, 18) = 1.5, p = 0.25 for treatment; F(2,18) = 1.4, p = 0.26 for interaction) or BERKO (F(1, 22) = 1.1, p = 0.31 for treatment; F(2, 22) = 1.4, p = 0.26 for interaction).
Thus, pAkt-ir increases after 6 hours of estradiol treatment in wild-type mice throughout the hippocampal formation, most prominently in the CA1 region. This effect is absent in AERKO and BERKO mice.
Estradiol affects PSD-95-ir in ER knockout mice
PSD-95-ir was found in areas containing neuropil with the densest labeling in dentate gyrus, and was completely absent from principal cell bodies (Fig. 3A-B). Two-way ANOVA showed a significant effect of subregion at both 6- and 48-hour time points (F(2, 34) = 36, p < 0.0001 for 6 hours; F(2, 34) = 72, p < 0.0001 for 48 hours) (Fig 3C). Post-hoc tests showed that PSD-95-ir was higher in dentate hilus than in CA1 or CA3 (p < 0.05 for both time points and treatment groups). PSD-95-ir was also higher in CA1 than CA3 for the estradiol-treated group at 6 hours, and for the vehicle-treated group at 48 hours (p < 0.05 for both). Estradiol treatment had no overall effect on PSD-95-ir at 6 hours (F(1, 34) = 0.53, p = 0.48), but there was a significant interaction between treatment and subregion (F(2, 34) = 4.5, p = 0.019). Indeed, estradiol-treated mice had noticeably more PSD-95-ir than vehicle-treated mice only in CA1 stratum radiatum, but this difference was not significant in post-hoc tests (p > 0.05 for each subregion). At 48 hours, there was no overall effect of treatment on PSD-95-ir (F(1, 34) = 0.13, p = 0.72), and no interaction between treatment and subregion (F(2, 34) = 0.89, p = 0.42). Thus, after 6 hours of treatment, estradiol seems to affect PSD-95-ir differently in different hippocampal subregions.
Fig. 3.
Micrographs (A-B) show PSD-95-ir in representative sections through the dorsal hippocampal formation of a female wild-type mouse treated with vehicle or estradiol for 6 hours (A) or 48 hours (B). Graphs (C) show the optical density of PSD-95-ir after 6 and 48 hours of vehicle or estradiol treatment in wild-type mice. Micrographs (D-E) show immunocytochemistry for pAkt in representative sections through the dorsal hippocampal formation of female AERKO (D) and BERKO (E) mice treated with estradiol for 6 hours. Graphs (F) show the optical density of PSD-95-ir after 6 hours of vehicle or estradiol treatment in AERKO or BERKO mice. Values from wild-type mice in AERKO and BERKO litters were pooled. Group “a” is significantly different from group “b,” p<0.05. N = 9 for wild-type, 5 for AERKO (vehicle), 6 for AERKO (estradiol), 7 for BERKO. Marker bar, 100 µm.
Based on the subregion×treatment interaction at the 6 hour time point, we analyzed PSD-95-ir in AERKO and BERKO mice treated with vehicle or estradiol for 6 hours to determine whether ER knockout alters the effect of estradiol on PSD-95-ir (Fig 3D-F). PSD-95-ir showed the same staining patter in AERKO and BERKO as in wild-type mice, with denser labeling in dentate hilus than in CA1 or CA3 stratum radiatum. Two-way ANOVA showed an overall effect of subregion for both AERKO (F(2, 18) = 22, p < 0.0001) and BERKO (F(2, 24) = 25, p < 0.0001). Post-hoc tests showed that both vehicle- and estradiol-treated groups had significantly more PSD-95-ir in the dentate hilus than in CA1 or CA3 stratum radiatum (p < 0.05 for both genotypes and treatment groups). Estradiol tended to increase PSD-95-ir in AERKO mice, but this trend was not statistically significant; two-way ANOVA showed a trend toward an overall effect of treatment (F(1, 18) = 3.4, p = 0. 099) and no interaction between treatment and subregion (F(2, 18) = 0.75, p = 0.49). In contrast, estradiol tended to decrease PSD-95 in BERKO mice; again, this trend was not statistically significant. Two-way ANOVA showed a trend toward an overall effect of treatment (F(1, 24) = 2.7, p = 0.13), and no interaction between treatment and subregion (F(2, 24) = 1.1, p = 0.34). Thus, estradiol tended to have opposite effects on PSD-95-ir in AERKO and BERKO mice, increasing PSD-ir in AERKOs while decreasing it in BERKO, but these effects were small and not statistically significant.
ER alpha or beta knockout does not affect baseline levels of pAkt-ir or PSD-95-ir
ERs may be important for estradiol effects on hippocampal signaling in adulthood, but they may also play a role in hippocampal development. AERKO or BERKO mice may therefore show differences in hippocampal architecture or function in adulthood, irrespective of the presence of estradiol, which could alter the effect of estradiol on the adult AERKO or BERKO hippocampus. We observed no differences in hippocampal architecture between wild-type, AERKO and BERKO mice, including the cytoarchitecture of hippocampal cell layers and pattern of pAkt-ir and PSD-95 labeling, suggesting that any such developmental disruptions are subtle. To probe further for any baseline differences in Akt signaling or PSD-95 expression among ER knockout mice, we compared pAkt-ir and PSD-95-ir in vehicle-treated AERKO or BERKO mice and their pooled wild-type littermates (Fig. 4). For pAkt-ir (Fig. 4A), AERKO mice seemed to have more pAkt-ir than wild-type mice in every hippocampal subregion, but this effect was not statistically significant. Two-way ANOVA showed an overall effect of subregion (F(2, 38) = 85, p < 0.0001), a trend toward an effect of genotype (F(2, 38) = 3.2, p = 0.063), and no interaction between genotype and subregion (F(4, 38) = 1.7, p = 0.16). For PSD-95-ir (Fig. 4B), two-way ANOVA showed an overall effect of subregion (F(2, 36) = 40.62, p < 0.0001), no overall effect of genotype (F(2, 36) = 0.83, p = 0.45), and no interaction between genotype and subregion (F(4, 36) = 0.50, p = 0.74). Therefore, ER alpha or beta knockout does not significantly alter the baseline levels of pAkt-ir or PSD-95-ir in ovariectomized, vehicle-treated mice.
Fig. 4.
Graph shows the optical density of pAkt-ir (A) and PSD-95-ir (B) in ovariectomized wild-type, AERKO, and BERKO mice after 6 hours vehicle treatment. N = 9 for wild-type, 5 for AERKO, 7 for BERKO.
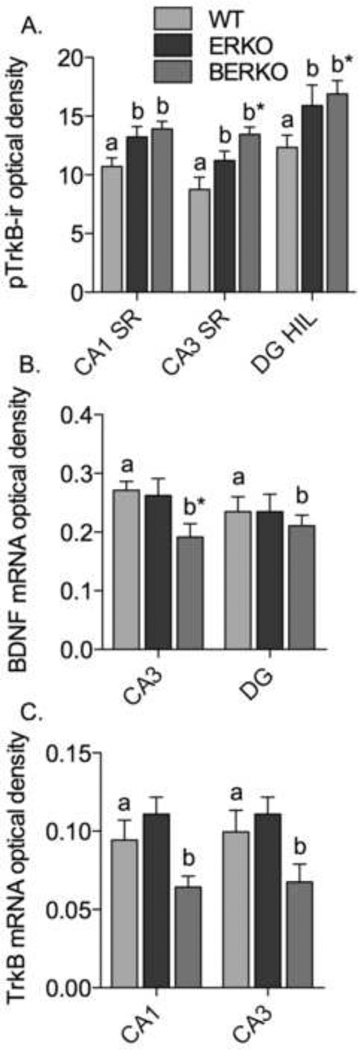
Estradiol treatment for 48 hours increases pTrkB-ir via ERs alpha and beta
pTrkB-ir was found in principal cell bodies and neuropil throughout the hippocampal formation (Fig. 5A-B). The densest pTrkB-ir was found in the hilus of the dentate gyrus: two-way ANOVA showed an overall effect of subregion for the 6 hour (F(2, 38) = 33, p < 0.0001) and 48 hour treatments (F(2, 30) = 7.0, p = 0.0033) (Fig. 5C). Post-hoc tests showed that pTrkB-ir was significantly higher in dentate hilus than in CA1 or CA3 stratum radiatum at the 6-hour time point (p < 0.01), and significantly higher in dentate hilus than in CA3 stratum radiatum for the 48 hour-vehicle treatment group. Six hours after estradiol treatment, there was no change in pTrkB-ir in comparison to vehicle treatment; two-way ANOVA showed no overall effect of treatment (F(1, 38) = 0.22, p = 0.65) and no interaction between treatment and subregion (F(2, 38) = 0.093, p = 0.91). In contrast, 48 hours of estradiol treatment increased pTrkB-ir in every hippocampal subregion: two-way ANOVA showed a significant overall effect of treatment (F(1, 30) = 7.7, p = 0.014), and no interaction between treatment and subregion (F(2, 30) = 1.1, p = 0.35). Post-hoc tests showed that estradiol treatment significantly increased pTrkB-ir in the CA3 stratum radiatum (p < 0.01).
Fig. 5.
Micrographs (A-B) show pTrkB-ir in representative sections through the dorsal hippocampal formation of a female wild-type mouse treated with vehicle or estradiol for 6 hours (A) or 48 hours (B). Graphs (C) show the optical density of pTrkB-ir after 6 and 48 hours of vehicle or estradiol treatment in wild-type mice. Micrographs (D-E) show immunocytochemistry for pTrkB in representative sections through the dorsal hippocampal formation of female AERKO (D) and BERKO (E) mice treated with estradiol for 48 hours. Graphs (F) show the optical density of pTrkB-ir after 48 hours of estradiol treatment in AERKO or BERKO mice. Group “a” is significantly different from group “b,” p<0.05.. * p < 0.05 relative to vehicle treatment. N = 10 (vehicle) and 11 (estradiol) for wild-type (6 hours), 9 (vehicle) and 8 (estradiol) for wild-type (48 hours), 6 for AERKO (vehicle), 7 for AERKO (estradiol), 5 for BERKO (vehicle), 4 for BERKO (estradiol). Marker bar, 100 µm.
To determine whether the increase in pTrkB-ir after 48 hours of estradiol treatment requires ER alpha or beta, pTrkB-ir was measured in AERKO and BERKO mice treated with estradiol or oil vehicle for 48 hours (Fig. 5D-F). pTrkB-ir showed the same staining pattern in AERKO and BERKO as in wild-type mice (Fig. 5D, E). Two-way ANOVA showed a significant overall effect of subregion for AERKO (F(2, 22) = 10.62, p = 0.0006), and a trend for BERKO (F(2, 14) = 3.6, p = 0.056). Post-hoc tests showed that pTrkB-ir was significantly higher in dentate than in CA3 in AERKO mice (p < 0.01 for both treatment groups). The lack of a significant effect of subregion in BERKO mice was likely due to the higher overall variability of labeling density and lower N for BERKO mice. Estradiol had no effect on pTrkB-ir in AERKO or BERKO mice; two-way ANOVA showed no overall effect of treatment and no interaction between treatment and subregion for AERKO (F(1, 22) = 0.040, p = 0.84 for treatment; F(2, 22) = 0.056, p = 0.95 for interaction) or BERKO (F(1, 14) = 0.49, p = 0.51 for treatment; F(2, 14) = 0.30, p = 0.75 for interaction). Thus, ERs alpha and beta are both required for estradiol to increase pTrkB-ir.
In contrast to the relatively rapid effect of estradiol on pAkt-ir, the longer treatment was required to increase pTrkB-ir, suggesting that this effect of estradiol is mediated via a different mechanism. Increased gene transcription and protein expression of TrkB itself or of the TrkB ligand, BDNF, could explain the increase in pTrkB-ir after estradiol treatment. To determine whether estradiol increases BDNF or TrkB transcription, we used in situ hybridization to identify BDNF and TrkB in the hippocampal formation of wild-type mice treated with vehicle or estradiol for 48 hours (Fig. 6).
Fig. 6.
Micrographs (A) show in situ hybridization for BDNF mRNA in representative sections through the dorsal hippocampal formation of wild-type female mice treated with vehicle or estradiol for 48 hours. Graph (B) shows optical density of BDNF mRNA in CA3 pyramidal cell layer and dentate granule cell layer from wild-type mice treated for 48 hours with vehicle or estradiol. Micrographs (C) show in situ hybridization for TrkB mRNA in representative sections through the dorsal hippocampal formation of wild-type mice treated with vehicle or estradiol for 48 hours. Graph (D) shows optical density of TrkB mRNA in CA1 and CA3 pyramidal cell layers from wild-type mice treated for 48 hours with vehicle or estradiol. N = 8 (vehicle) and 9 (estradiol) for BDNF, 9 (vehicle) and 6 (estradiol) for TrkB. Marker bars, 100 µm.
BDNF and TrkB mRNA were found in all hippocampal principal cell layers: CA1 and CA3 pyramidal cell layers, and dentate granule cell layer (Fig 6A and C). In the CA1 pyramidal cell layer, the optical density of BDNF mRNA was not increased above background in several sections, and so this region was excluded from the final analysis. For the same reason, the dentate granule cell layer was excluded from the final analysis of TrkB mRNA. BDNF mRNA was slightly increased in CA3 over dentate for both treatment groups (Fig. 6B), two-way ANOVA showed an overall effect of subregion (F(1, 15) = 15, p = 0.0016). TrkB mRNA was of a similar density in both CA1 and CA3 (Fig. 6C); two-way ANOVA showed no overall effect of subregion (F(1, 13) = 1.3, p = 0.77). Estradiol-treated animals had more BDNF mRNA in CA3 and dentate principal cell layers compared to vehicle treatment (Fig. 6B): two-way ANOVA showed a trend toward an overall effect of treatment on BDNF mRNA (F(1, 15) = 2.7, p = 0.12), and no interaction between treatment and subregion (F(1, 15) = 0.42, p = 0.53). Estradiol treatment had no effect on TrkB mRNA (Fig. 6D); two-way ANOVA showed no overall effect of treatment (F(1, 13) = 0.10, p = 0.76) and no interaction between treatment and subregion (F(1, 13) = 0.089, p = 0.77).
Based on the finding of a trend toward increased BDNF mRNA with 48 hours of estradiol treatment in wild-type mice, BDNF mRNA was assayed using in situ hybridization in AERKO and BERKO mice after 48 hours of estradiol or vehicle treatment (Fig. 7). The pattern of BDNF mRNA was similar to that of wild-type mice, with minimal labeling in CA1 pyramidal cell layer and similarly dense labeling in CA3 and dentate principle cell layers. Two-way ANOVA showed a significant effect of subregion only for BERKO mice (F(1, 11) = 1.8, p = 0.20 for AERKO; (F(1, 10) = 11, p = 0.0072 for BERKO), with no significant post-hoc tests. Estradiol-treated AERKO mice had more BDNF mRNA than vehicle-treated mice in both CA3 and dentate; two-way ANOVA showed a significant effect of estradiol treatment (F(1, 11) = 8.7, p = 0.013), with no interaction between treatment and subregion (F(1, 11) = 0.013, p = 0.91). Post-hoc tests showed that estradiol significantly increased BDNF mRNA in the CA3 pyramidal cell layer (p < 0.05). In contrast, estradiol had no effect on BDNF mRNA in BERKO mice; two-way ANOVA showed no overall effect of treatment (F(1, 10) = 0.063, P = 0.81) and no interaction between treatment and subregion (F(1, 10) = 0.0046, p = 0.95). Thus, estradiol tends to increase BDNF mRNA in wild-type mice and does so significantly in AERKO mice.
Fig. 7.
Graphs show the optical density of BDNF mRNA in CA3 pyramidal cell layer and dentate granule cell layers of AERKO and BERKO mice treated with vehicle or estradiol for 48 hours. Group “a” is significantly different from group “b,” p<0.05. * p < 0.05 relative to vehicle treatment. N = 7 for AERKO (vehicle), 6 for AERKO (estradiol), 6 for BERKO.
ER knockout alters baseline levels of pTrkB-ir
As mentioned above for pAkt and PSD-95, differences in baseline levels of BDNF and TrkB signaling, possibly due to developmental differences in wild-type and ER knockout animals, could alter the response of the hippocampal formation to estradiol. To investigate differences in baseline BDNF and TrkB signaling, pTrkB-ir, BDNF mRNA, and TrkB mRNA were compared among vehicle-treated wild-type, AERKO, and BERKO mice (Fig. 8). Ovariectomized AERKO and BERKO mice had significantly more pTrkB-ir than wild-type mice in every hippocampal subregion; two-way ANOVA showed a significant overall effect of genotype (F(2, 34) = 14, p = 0.0003) and subregion (F(2, 34) = 11, p = 0.0002), and no interaction between genotype and subregion (F(4, 34) = 0.19, p = 0.94). Post-hoc comparisons showed a significant increase in pTrkB-ir in all three subregions in AERKO and BERKO compared to wild-type mice (p < 0.05 for all). BERKO mice had less BDNF mRNA and TrkB mRNA than wild-type mice, but this difference was not statistically significant. For BDNF mRNA, two-way ANOVA showed no effect of genotype (F(2, 18) = 1.7, p = 0.20), subregion (F(1, 18) = 1.1, p = 0.30), or interaction between genotype and subregion (F(2, 18) = 1.5, p = 0.26). For TrkB mRNA, two-way ANOVA showed no effect of genotype (F(2, 16) = 2.0, p = 0.16), subregion (F(1, 16) = 0.56, p = 0.46), or interaction between genotype and subregion (F(2, 16) = 2.4, 0.12).
Fig. 8.
Graphs show the optical density of pTrkB-ir (A), BDNF mRNA (B), and TrkB mRNA (C) in the hippocampal formation of ovariectomized wild-type, AERKO, and BERKO mice after 48 hours vehicle treatment. * p < 0.05 relative to WT. N = 9 for wild-type, 6 for AERKO, 5 for BERKO.
Discussion
In humans, the hippocampal formation has been implicated in changes in mood and memory associated with fluctuations in circulating estrogen, across the menstrual cycle and after estrogenic therapies for breast cancer (Eberling et al., 2004, Protopopescu et al., 2008a, Protopopescu et al., 2008b). Dissecting how estrogens act in this brain region, through which receptors and modes of signaling, will enable us to explain and predict the effects of natural and pharmacologic estrogens in different clinical circumstances (Zhou et al., 2002, Bernardi et al., 2003, Ciriza et al., 2004, O'Neill et al., 2004, Rhodes and Frye, 2006, Zhao and Brinton, 2006). Thus understanding the molecular mechanisms by which estrogens affect hippocampal function has broad implications for women’s health.
In this study, we demonstrate temporally distinct, ER-dependent effects of estradiol treatment on two molecular markers of synaptic plasticity, phosphorylated Akt and phosphorylated TrkB. Estradiol treatment for 6 hours increased pAkt-ir, while estradiol treatment for 48 hours increased pTrkB-ir, throughout the female mouse hippocampus. These effects of estradiol were eliminated in AERKO and BERKO mice, and therefore require ERs alpha and beta. This is one of the first demonstrations of ER involvement in vivo on specific molecular pathways important for hippocampal synaptic plasticity. The influence of estradiol on these pathways may directly account for its effects on dendritic spine density, as Akt and TrkB signaling have both been implicated in estradiol-mediated increases in filopodia and dendritic spine formation in vitro (Akama and McEwen, 2003, Scharfman et al., 2003, Yuen, 2006, Sato et al., 2007).
Estradiol acts via multiple mechanisms to influence hippocampal signaling
Previous studies showed that estradiol activates kinase cascades and influences synaptic protein expression on different time courses in the same in vitro hippocampal neurons and cell lines (Akama and McEwen, 2003, Lee et al., 2004, Yuen et al., 2011). The current study is the first to demonstrate temporally distinct effects of estradiol treatment in vivo. On the one hand, estradiol increased pAkt-ir relatively rapidly, after just 6 hours of estradiol treatment. This time course is consistent with previous studies in female mice and hippocampal neurons in culture, and lends support to the idea that estradiol acts via membrane-affiliated receptors to phosphorylate Akt (Akama and McEwen, 2003, Lee et al., 2004, Fan et al., 2010). Indeed, both ERs alpha and beta are present at extranuclear sites in the mouse hippocampal formation (Mitterling et al., 2010), where they are well placed to carry out membrane-initiated activation of kinases such as Akt. Although this finding does not exclude the possibility that estradiol increases total Akt protein, previous work showed that estradiol increases pAkt without affecting the level of total Akt (Akama and McEwen, 2003, Fan et al., 2010).
In contrast to estradiol’s effect on pAkt, a longer treatment of 48 hours was necessary to increase BDNF signaling, demonstrated by an increase in pTrkB-ir. The time course of this effect suggests that it requires new gene transcription. It could not be explained by increased TrkB expression, since estradiol had no effect on TrkB mRNA. Increased BNDF gene transcription could play a role, since there was a trend toward increased BDNF mRNA with estradiol treatment. But increased BDNF was not sufficient for estradiol to increase pTrkB-ir, since the increase in BDNF mRNA in AERKO mice was not accompanied by a change in pTrkB-ir. Thus we find it more likely that estradiol affects BDNF mRNA and pTrkB-ir through independent mechanisms. It may increase pTrkB-ir by inducing transcription of genes encoding proteins involved in BDNF transport or release, as estradiol enhanced these processes in previous studies (Jezierski and Sohrabji, 2003, Sato et al., 2007). On the other hand, our findings do not exclude the possibility of post-transcriptional regulation of BDNF or TrkB expression, since BDNF and unphosphorylated TrkB proteins were not measured.
Estradiol’s effects on BDNF mRNA and pTrkB-ir at 48 hours could be mediated via nuclear ER signaling. For example, a putative ERE has been identified in the rat, mouse, and human BDNF gene that binds estrogen receptor-ligand complexes in vitro (Sohrabji et al., 1995). Alternatively, they may be downstream effects of membrane-initiated ER signaling. This seems likely, given the predominantly extranuclear location of ERs in the hippocampal formation (Mitterling et al., 2010). Moreover, we recently showed using electron microscopy that in proestrus, increased phosphorylation of a specific subset of axonal TrkB occurs (Spencer-Segal et al., 2011). If the increased pTrkB-ir after estradiol treatment also occurs primarily in axons, this suggests a local effect of ERs on axonal TrkB activation or trafficking. Finally, estradiol could increase translation of BDNF, TrkB, or other proteins involved in BDNF signaling via membrane-initiated actions on the cellular machinery for protein translation, as in NG108-15 cells (Akama and McEwen, 2003). Future studies should aim to distinguish among these possibilities.
Hippocampal estradiol signaling is balanced between ERs alpha and beta
This is the first in vivo study to extensively compare the effects of ERs alpha and beta on hippocampal estradiol signaling. The importance of ER alpha for estradiol activation of Akt is not surprising, as ER alpha binds in vitro to the p85 regulatory subunit of Akt’s upstream activator, PI3K, in rat primary cortical neurons (Mannella and Brinton, 2006). Recent studies also demonstrated ER beta’s importance in hippocampal CREB signaling, synaptic protein expression, and spatial memory (Abraham et al., 2003, Rhodes and Frye, 2006, Liu et al., 2008). The current study tempers the emphasis on one or another ER for hippocampal function. Intriguingly, it shows that the two ERs alpha and beta do not always have distinct effects, but that they have complementary roles in increasing pAkt and pTrkB after estradiol administration.
ERs alpha and beta may cooperate via several possible mechanisms to increase pAkt and pTrkB after estradiol treatment. They may form heterodimers as has been demonstrated in vitro (Pettersson et al., 1997) and in so doing activate signaling pathways distinct from those activated by ER alpha and beta homodimers. It is not known whether ERs alpha and beta are co-expressed in the same cells in the adult hippocampus, which would be required for such heterodimers signaling to occur. Alternatively, the two receptors may activate distinct upstream signaling pathways – in the same or different cells – that converge on a final result of increased Akt of TrkB phosphorylation.
On their own, ERs alpha and beta can have antagonizing effects on gene transcription (Matthews and Gustafsson, 2003). Our findings suggest that this phenomenon occurs in the hippocampus. For example, estradiol caused trends toward opposite effects on PSD-95-ir, producing an increase in AERKOs, a decrease in BERKOs, and no main effect in wild-type mice. This system of opposition could serve to limit the extent of estradiol-induced excitability, particularly important in the hippocampal formation given the vulnerability of this brain region to excitotoxicity and seizure.
The data therefore indicate both ERs alpha and beta are required for estradiol effects on pathways important for synaptic plasticity in the mouse hippocampal formation, and that the two receptors have complementary and distinct effects.
Relationship to estrous cycle changes in signaling pathways
In previous studies, we showed that pAkt-ir and pTrkB-ir increase in parallel during proestrus, without the temporal separation seen here with estradiol treatment (Znamensky et al., 2003, Spencer et al., 2008a, Spencer et al., 2010). In those studies there also was a corresponding increase in PSD-95-ir and BDNF mRNA during proestrus, which here were not statistically significant in wild-type estradiol-treated mice (Spencer et al., 2008a, Spencer et al., 2010).
The discrepancies between this and our previous studies could be related to fluctuations in other hormones such as progesterone and the gonadotrophins across the estrous cycle, or the timing of estradiol exposure. In the proestrus mice, estradiol had likely been elevated for 6-24 hours, but the kinetics of its rise and fall in proestrus are very different from the more uniformly increased level of circulating estradiol that arises from an injection of estradiol benzoate. We hypothesize that the rise and fall of circulating estradiol across the estrous cycle optimizes the integration of multiple estradiol effects (Hammes and Levin, 2007), resulting in the convergence of different estradiol effects on the late morning of proestrus.
How the fluctuations of estradiol or other hormones across the cycle may be timed to elicit specific effects is as of yet unclear. It could involve an integration of membrane and nuclear ER signaling: examples of this integration can be found in numerous cell types, where rapid estradiol-mediated phosphorylation of transcription factors and ERs themselves facilitates nucleus-initiated signaling with appropriately timed hormone administration (Vasudevan et al., 2001, Levin, 2005, Madak-Erdogan et al., 2008). Indeed, estradiol likely increases PSD-95 expression in hippocampus via both membrane- and nucleus-mediated signaling (Akama and McEwen, 2003, Sato et al., 2007)(Akama, unpublished observations). On the other hand, natural fluctuations in estradiol and other hormones may more effectively target certain ERs. In the current study, estradiol did increase PSD-95-ir and BDNF mRNA in the AERKO mice, effects that were not seen in wild-type. Perhaps the absence of ER alpha unmasked actions of estradiol through ER beta or other estrogen receptors (Madak-Erdogan et al., 2008, Hammond and Gibbs, 2010) that are naturally elicited during proestrus. This puzzle is an important one, as it likely has important consequences for hippocampal function: one recent study showed that precise timing of increased circulating estradiol was important for the increase in hippocampal cell excitability seen during proestrus (Scharfman et al., 2007).
Effects of ER knockout on hippocampal development
The differences between wild-type and AERKO or BERKO mice represent a combination of the developmental effects of ER knockout and the absence of a given ER during adulthood. It is difficult to determine whether disruptions in E signaling in AERKO and BERKO mice result from the absence of ER per se, or from a disruption of E effects due to compensatory changes in brain development and function. To determine whether a disruption in the cellular environment occurs with ER knockout that might account in part for the difference in estrogen sensitivity in AERKO and BERKO mice, we compared the staining pattern and examined baseline levels of each endpoint in ovariectomized, vehicle-treated mice. This experiment has significant limitations, as the results may be measuring the effect of vehicle injection and/or ovariectomy and may not correlate with intact mice with low levels of circulating estradiol. With this caveat in mind, we found comparable staining patterns and no change in pAkt-ir, PSD-95-ir, or BDNF mRNA in AERKO and BERKO mice, suggesting that baseline hippocampal architecture and function is generally preserved in these knockouts. On the other hand, the finding of elevated pTrkB-ir in AERKO and BERKO mice suggests that ER knockout during development indeed alters the environment in which ERs signal in adulthood. This increase in pTrkB-ir in AERKO and BERKO mice suggests that ERs alpha and beta work to maintain an appropriate level of neurotrophin signaling in the presence and absence of circulating estradiol. ERs alpha and beta may carry out these roles via ligand-independent ER signaling (Coleman and Smith, 2001), or via activation by steroids synthesized locally in the brain (Kuppers and Beyer, 1998, Hojo et al., 2004, Kretz et al., 2004).
In AERKO mice, a lack of feedback inhibition of gonadotropin secretion causes persistent elevations in circulating estradiol (and potentially other hormonal perturbations) that could influence hippocampal function (Couse et al., 1995). Increased exposure to estradiol during development could itself cause the elevated basal level of pTrkB-ir in AERKO mice, since estradiol increases hippocampal pTrkB-ir. Alternatively, precipitous withdrawal of estradiol stimulation after ovariectomy may impact the hippocampal formation differently in mice accustomed to higher levels of circulating estradiol, thereby altering the baseline levels of endpoints in this study. Because we only examined hippocampi from ovariectomized AERKO and wild-type mice, we do not know whether the alteration in pTrkB-ir in vehicle-treated ER knockout mice represents a divergent response to ovariectomy, or whether these differences are also seen in intact mice. Finally, the absence of an effect of estradiol on hippocampal signaling and gene expression could be due to a relative desensitization of the AERKO hippocampal formation to lower concentrations of circulating estradiol, rather than a lack of estradiol signaling mechanisms per se. Although circulating estradiol is not increased in BERKO mice, differences in the levels of other circulating hormones such as LH and corticosterone may also affect the development of the hippocampus, and may contribute to the elevated baseline pTrkB-ir or other effects seen in this study (Dorling et al., 2003, Walf et al., 2009).
Altered baseline levels of pTrkB-ir in ER knockout mice may also result from the lack of ERs during hippocampal development. ERs alpha and beta are both expressed in the neonatal hippocampus (Su et al., 2001). Estradiol may affect CNS development via its modulation of neurotrophin signaling, suggested by the ability of gonadectomy and estradiol replacement to alter the expression levels of BDNF in the developing rat brain (Solum and Handa, 2002). The absence of ER alpha or beta may also change the expression pattern of other ERs and estrogen-related receptors in the brain, altering the way estradiol modulates neurotrophin signaling.
The possibility of differences in hippocampal development in AERKO and BERKO mice are an additionally interesting avenue for future research on the role of ERs in normal brain development and the effects of abnormal sex steroid exposure on adult behavior (Imwalle et al., 2006). Given this caveat to our current findings, future studies should further investigate the effects of ERs alpha and beta using conditional knockouts or selective ER agonists.
Functional considerations
Evidence is accumulating that synaptic plasticity such as that mediated by Akt and TrkB in the hippocampus underlies learning and memory (Engert and Bonhoeffer, 1999, Minichiello et al., 1999, Whitlock et al., 2006, Costa-Mattioli et al., 2009, Fan et al., 2010). Estradiol effects on Akt and TrkB are therefore likely to be important for its influence on hippocampal-dependent cognition. Increased Akt and TrkB signaling would theoretically facilitate synaptic plasticity, leading to an expected increase in hippocampal-dependent memory in estradiol-treated mice. But it is not possible to predict the effect of 6 or 48 hours of estradiol treatment on hippocampal-dependent learning and memory in mice based solely on these findings. In our recent study of naturally cycling wild-type mice, spatial memory was at its best during diestrus, when hippocampal pAkt-ir, BDNF mRNA, and pTrkB-ir were low (Spencer et al., 2010). Thus the relationship between these endpoints and spatial memory is not yet well defined. This is not surprising, as years of experience have taught us that the effects of estradiol on hippocampal-dependent behaviors are complex and often unpredictable (Frick, 2009). In the long run, we predict that estradiol activation of ERs alpha and beta mediates the effects of endogenous and exogenous estrogens on behaviors involving the hippocampus at least in part via Akt and TrkB signaling.
Highlights.
We examined the effect of estradiol treatment on Akt and BDNF signaling in the mouse hippocampus.
6 hours of estradiol treatment increased pAkt immunoreactivity, while 48 hours increased pTrkB immunoreactivity.
Estradiol effects were all blocked by knockout of estrogen receptor beta.
Estradiol effects on Akt and TrkB were blocked by knockout of estrogen receptor alpha.
Acknowledgements
The authors would like to acknowledge Dr. Diane Lane at Weill Cornell Medical College for valuable advice on statistical analysis.
Support for this work was provided by NIH grants MH082528 and GM07739 (JLS), DA08259 (TAM), DK07313 (EMW), NS007080 and AG016765 (BSM), and JSPS grants 17052001 and 20022005 (SO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT: The authors have nothing to disclose.
References
- Abraham IM, Han SK, Todman MG, Korach KS, Herbison AE. Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- Adams MM, Fink SE, Shah RA, Janssen WG, Hayashi S, Milner TA, McEwen BS, Morrison JH. Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats. J Neurosci. 2002;22:3608–3614. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MM, Shah RA, Janssen WG, Morrison JH. Different modes of hippocampal plasticity in response to estrogen in young and aged female rats. Proc Natl Acad Sci U S A. 2001;98:8071–8076. doi: 10.1073/pnas.141215898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akama KT, McEwen BS. Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway. J Neurosci. 2003;23:2333–2339. doi: 10.1523/JNEUROSCI.23-06-02333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalo JC, Waite J, Rajagopal R, Beyna M, Chen ZY, Lee FS, Chao MV. Cell survival through Trk neurotrophin receptors is differentially regulated by ubiquitination. Neuron. 2006;50:549–559. doi: 10.1016/j.neuron.2006.03.044. [DOI] [PubMed] [Google Scholar]
- Bath KG, Mandairon N, Jing D, Rajagopal R, Kapoor R, Chen ZY, Khan T, Proenca CC, Kraemer R, Cleland TA, Hempstead BL, Chao MV, Lee FS. Variant brain-derived neurotrophic factor (Val66Met) alters adult olfactory bulb neurogenesis and spontaneous olfactory discrimination. J Neurosci. 2008;28:2383–2393. doi: 10.1523/JNEUROSCI.4387-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Katche C, Slipczuk L, Rossato JI, Goldin A, Izquierdo I, Medina JH. BDNF is essential to promote persistence of long-term memory storage. Proc Natl Acad Sci U S A. 2008;105:2711–2716. doi: 10.1073/pnas.0711863105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi F, Pluchino N, Stomati M, Pieri M, Genazzani AR. CNS: sex steroids and SERMs. Ann N Y Acad Sci. 2003;997:378–388. doi: 10.1196/annals.1290.041. [DOI] [PubMed] [Google Scholar]
- Brown CM, Mulcahey TA, Filipek NC, Wise PM. Production of proinflammatory cytokines and chemokines during neuroinflammation: novel roles for estrogen receptors alpha and beta. Endocrinology. 2010;151:4916–4925. doi: 10.1210/en.2010-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PC, Aicher SA, Drake CT. Kappa opioid receptors in rat spinal cord vary across the estrous cycle. Brain Res. 2000;861:168–172. doi: 10.1016/s0006-8993(99)02461-0. [DOI] [PubMed] [Google Scholar]
- Ciriza I, Carrero P, Azcoitia I, Lundeen SG, Garcia-Segura LM. Selective estrogen receptor modulators protect hippocampal neurons from kainic acid excitotoxicity: differences with the effect of estradiol. J Neurobiol. 2004;61:209–221. doi: 10.1002/neu.20043. [DOI] [PubMed] [Google Scholar]
- Coleman KM, Smith CL. Intracellular signaling pathways: nongenomic actions of estrogens and ligand-independent activation of estrogen receptors. Front Biosci. 2001;6:D1379–D1391. doi: 10.2741/coleman. [DOI] [PubMed] [Google Scholar]
- Cordoba Montoya DA, Carrer HF. Estrogen facilitates induction of long term potentiation in the hippocampus of awake rats. Brain Res. 1997;778:430–438. doi: 10.1016/s0006-8993(97)01206-7. [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Curtis SW, Washburn TF, Lindzey J, Golding TS, Lubahn DB, Smithies O, Korach KS. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol Endocrinol. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm Behav. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Day M, Sung A, Logue S, Bowlby M, Arias R. Beta estrogen receptor knockout (BERKO) mice present attenuated hippocampal CA1 long-term potentiation and related memory deficits in contextual fear conditioning. Behav Brain Res. 2005;164:128–131. doi: 10.1016/j.bbr.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Dorling AA, Todman MG, Korach KS, Herbison AE. Critical role for estrogen receptor alpha in negative feedback regulation of gonadotropin-releasing hormone mRNA expression in the female mouse. Neuroendocrinology. 2003;78:204–209. doi: 10.1159/000073703. [DOI] [PubMed] [Google Scholar]
- Eberling JL, Wu C, Tong-Turnbeaugh R, Jagust WJ. Estrogen- and tamoxifen-associated effects on brain structure and function. Neuroimage. 2004;21:364–371. doi: 10.1016/j.neuroimage.2003.08.037. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Klein M, Rumpel S, Malinow R. PSD-95 is required for activity- driven synapse stabilization. Proc Natl Acad Sci U S A. 2007;104:4176–4181. doi: 10.1073/pnas.0609307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J Neurosci. 2010;30:4390–4400. doi: 10.1523/JNEUROSCI.4333-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy MR, Baudry M, Foy JG, Thompson RF. 17beta-estradiol modifies stress-induced and age-related changes in hippocampal synaptic plasticity. Behav Neurosci. 2008;122:301–309. doi: 10.1037/0735-7044.122.2.301. [DOI] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- Frick KM. Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm Behav. 2009;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Berger-Sweeney J. Spatial reference memory and neocortical neurochemistry vary with the estrous cycle in C57BL/6 mice. Behav Neurosci. 2001;115:229–237. doi: 10.1037/0735-7044.115.1.229. [DOI] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugger HN, Foster TC, Gustafsson J, Rissman EF. Novel effects of estradiol and estrogen receptor alpha and beta on cognitive function. Brain Res. 2000;883:258–264. doi: 10.1016/s0006-8993(00)02993-0. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Levels of trkA and BDNF mRNA, but not NGF mRNA, fluctuate across the estrous cycle and increase in response to acute hormone replacement. Brain Res. 1998;787:259–268. doi: 10.1016/s0006-8993(97)01511-4. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos I, Alejandre-Gomez M, Cervantes M. Spine-type densities of hippocampal CA1 neurons vary in proestrus and estrus rats. Neurosci Lett. 2005;379:52–54. doi: 10.1016/j.neulet.2004.12.043. [DOI] [PubMed] [Google Scholar]
- Good M, Day M, Muir JL. Cyclical changes in endogenous levels of oestrogen modulate the induction of LTD and LTP in the hippocampal CA1 region. Eur J Neurosci. 1999;11:4476–4480. doi: 10.1046/j.1460-9568.1999.00920.x. [DOI] [PubMed] [Google Scholar]
- Gottfried-Blackmore A, Croft G, Clark J, McEwen BS, Jellinck PH, Bulloch K. Characterization of a cerebellar granule progenitor cell line, EtC.1, and its responsiveness to 17-beta-estradiol. Brain Res. 2007;1186:29–40. doi: 10.1016/j.brainres.2007.08.071. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav. 2006;84:112–119. doi: 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocr Rev. 2007;28:726–741. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- Hammond R, Gibbs RB. GPR30 is positioned to mediate estrogen effects on basal forebrain cholinergic neurons and cognitive performance. Brain Res. 2010 doi: 10.1016/j.brainres.2010.11.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci U S A. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongpaisan J, Alkon DL. A structural basis for enhancement of long-term associative memory in single dendritic spines regulated by PKC. Proc Natl Acad Sci U S A. 2007;104:19571–19576. doi: 10.1073/pnas.0709311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RG, Vicentic A, Rogge G, Kuhar MJ. The effects of cocaine on CART expression in the rat nucleus accumbens: a possible role for corticosterone. Eur J Pharmacol. 2005;517:45–50. doi: 10.1016/j.ejphar.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Imwalle DB, Bateman HL, Wills A, Honda S, Harada N, Rissman EF. Impairment of spatial learning by estradiol treatment in female mice is attenuated by estradiol exposure during development. Horm Behav. 2006;50:693–698. doi: 10.1016/j.yhbeh.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Jezierski MK, Sohrabji F. Estrogen enhances retrograde transport of brain- derived neurotrophic factor in the rodent forebrain. Endocrinology. 2003;144:5022–5029. doi: 10.1210/en.2003-0724. [DOI] [PubMed] [Google Scholar]
- Joffe H, Cohen LS, Harlow BL. Impact of oral contraceptive pill use on premenstrual mood: predictors of improvement and deterioration. Am J Obstet Gynecol. 2003;189:1523–1530. doi: 10.1016/s0002-9378(03)00927-x. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Kim MT, Soussou W, Gholmieh G, Ahuja A, Tanguay A, Berger TW, Brinton RD. 17beta-Estradiol potentiates field excitatory postsynaptic potentials within each subfield of the hippocampus with greatest potentiation of the associational/commissural afferents of CA3. Neuroscience. 2006;141:391–406. doi: 10.1016/j.neuroscience.2006.03.075. [DOI] [PubMed] [Google Scholar]
- Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci. 2002;116:411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J. Shifts in preferred learning strategy across the estrous cycle in female rats. Horm Behav. 2004;45:330–338. doi: 10.1016/j.yhbeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 2004;24:5913–5921. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppers E, Beyer C. Expression of aromatase in the embryonic and postnatal mouse striatum. Brain Res Mol Brain Res. 1998;63:184–188. doi: 10.1016/s0169-328x(98)00279-4. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Campomanes CR, Sikat PT, Greenfield AT, Allen PB, McEwen BS. Estrogen induces phosphorylation of cyclic AMP response element binding (pCREB) in primary hippocampal cells in a time-dependent manner. Neuroscience. 2004;124:549–560. doi: 10.1016/j.neuroscience.2003.11.035. [DOI] [PubMed] [Google Scholar]
- Leuner B, Falduto J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. J Neurosci. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci U S A. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav. 1998;34:149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- Madak-Erdogan Z, Kieser KJ, Kim SH, Komm B, Katzenellenbogen JA, Katzenellenbogen BS. Nuclear and extranuclear pathway inputs in the regulation of global gene expression by estrogen receptors. Mol Endocrinol. 2008;22:2116–2127. doi: 10.1210/me.2008-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella P, Brinton RD. Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: a unified mechanism of estrogen action. J Neurosci. 2006;26:9439–9447. doi: 10.1523/JNEUROSCI.1443-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Milner TA. Hippocampal formation: Shedding light on the influence of sex and stress on the brain. Brain Res Rev. 2007 doi: 10.1016/j.brainresrev.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi E, Ying SW, Kanhema T, Croll SD, Bramham CR. Brain- derived neurotrophic factor triggers transcription-dependent, late phase long-term potentiation in vivo. J Neurosci. 2002;22:7453–7461. doi: 10.1523/JNEUROSCI.22-17-07453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minichiello L, Korte M, Wolfer D, Kuhn R, Unsicker K, Cestari V, Rossi- Arnaud C, Lipp HP, Bonhoeffer T, Klein R. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron. 1999;24:401–414. doi: 10.1016/s0896-6273(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Mitterling K, Spencer JL, Dziedzic N, Shenoy S, McCarthy K, Waters EM, McEwen B, Milner TA. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J Comp Neurol. 2010 doi: 10.1002/cne.22361. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D, Toni N, Buchs PA. Spine changes associated with long-term potentiation. Hippocampus. 2000;10:596–604. doi: 10.1002/1098-1063(2000)10:5<596::AID-HIPO10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Segal M. Regulation of dendritic spine density in cultured rat hippocampal neurons by steroid hormones. J Neurosci. 1996;16:4059–4068. doi: 10.1523/JNEUROSCI.16-13-04059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- O'Neill K, Chen S, Diaz Brinton R. Impact of the selective estrogen receptor modulator, tamoxifen, on neuronal outgrowth and survival following toxic insults associated with aging and Alzheimer's disease. Exp Neurol. 2004;188:268–278. doi: 10.1016/j.expneurol.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. Neuroreport. 1997a;8:3009–3013. doi: 10.1097/00001756-199709290-00004. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Posttraining estradiol injections enhance memory in ovariectomized rats: cholinergic blockade and synergism. Neurobiol Learn Mem. 1997b;68:172–188. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press, An Imprint of Elsevier; 1998. [Google Scholar]
- Pettersson K, Grandien K, Kuiper GG, Gustafsson JA. Mouse estrogen receptor beta forms estrogen response element-binding heterodimers with estrogen receptor alpha. Mol Endocrinol. 1997;11:1486–1496. doi: 10.1210/mend.11.10.9989. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Kurucz OS, Milner TA. Morphometry of a peptidergic transmitter system: dynorphin B-like immunoreactivity in the rat hippocampal mossy fiber pathway before and after seizures. Hippocampus. 1999;9:255–276. doi: 10.1002/(SICI)1098-1063(1999)9:3<255::AID-HIPO6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Butler T, Pan H, Root J, Altemus M, Polanecsky M, McEwen B, Silbersweig D, Stern E. Hippocampal structural changes across the menstrual cycle. Hippocampus. 2008a;18:985–988. doi: 10.1002/hipo.20468. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Tuescher O, Pan H, Epstein J, Root J, Chang L, Altemus M, Polanecsky M, McEwen B, Stern E, Silbersweig D. Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord. 2008b;108:87–94. doi: 10.1016/j.jad.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Register TC, Shively CA, Lewis CE. Expression of estrogen receptor alpha and beta transcripts in female monkey hippocampus and hypothalamus. Brain Res. 1998;788:320–322. doi: 10.1016/s0006-8993(98)00036-5. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. ERbeta-selective SERMs produce mnemonic- enhancing effects in the inhibitory avoidance and water maze tasks. Neurobiol Learn Mem. 2006;85:183–191. doi: 10.1016/j.nlm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Heck AL, Leonard JE, Shupnik MA, Gustafsson JA. Disruption of estrogen receptor beta gene impairs spatial learning in female mice. Proc Natl Acad SciU S A. 2002;99:3996–4001. doi: 10.1073/pnas.012032699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Horm Behav. 2004;45:128–135. doi: 10.1016/j.yhbeh.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Sanna PP, Cammalleri M, Berton F, Simpson C, Lutjens R, Bloom FE, Francesconi W. Phosphatidylinositol 3-kinase is required for the expression but not for the induction or the maintenance of long-term potentiation in the hippocampal CA1 region. J Neurosci. 2002;22:3359–3365. doi: 10.1523/JNEUROSCI.22-09-03359.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Akaishi T, Matsuki N, Ohno Y, Nakazawa K. beta-Estradiol induces synaptogenesis in the hippocampus by enhancing brain-derived neurotrophic factor release from dentate gyrus granule cells. Brain Res. 2007;1150:108–120. doi: 10.1016/j.brainres.2007.02.093. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Hintz TM, Gomez J, Stormes KA, Barouk S, Malthankar- Phatak GH, McCloskey DP, Luine VN, Maclusky NJ. Changes in hippocampal function of ovariectomized rats after sequential low doses of estradiol to simulate the preovulatory estrogen surge. Eur J Neurosci. 2007;26:2595–2612. doi: 10.1111/j.1460-9568.2007.05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. The influence of gonadal hormones on neuronal excitability, seizures, and epilepsy in the female. Epilepsia. 2006;47:1423–1440. doi: 10.1111/j.1528-1167.2006.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641–11652. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive functioning in women. Endocr Rev. 2003;24:133–151. doi: 10.1210/er.2001-0016. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Millard WJ, Simpkins JW. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res. 1994;644:305–312. doi: 10.1016/0006-8993(94)91694-2. [DOI] [PubMed] [Google Scholar]
- Smith CC, McMahon LL. Estradiol-induced increase in the magnitude of long-term potentiation is prevented by blocking NR2B-containing receptors. J Neurosci. 2006;26:8517–8522. doi: 10.1523/JNEUROSCI.5279-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solum DT, Handa RJ. Estrogen regulates the development of brain- derived neurotrophic factor mRNA and protein in the rat hippocampus. J Neurosci. 2002;22:2650–2659. doi: 10.1523/JNEUROSCI.22-07-02650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Milner TA, Lee FS, McEwen BS. BDNF variant Val66Met interacts with estrous cycle in the control of hippocampal function. Proc Natl Acad Sci U S A. 2010;107:4395–4400. doi: 10.1073/pnas.0915105107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Milner TA, McEwen BS. Estrous cycle regulates activation of hippocampal Akt, LIM kinase, and neurotrophin receptors in C57BL/6 mice. Neuroscience. 2008a;155:1106–1119. doi: 10.1016/j.neuroscience.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008b;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer-Segal JL, Waters EM, Bath KG, Chao MV, McEwen BS, Milner TA. Distribution of TrkB receptor in the mouse hippocampal formation depends on sex and estrous cycle stage. J Neurosci. 2011 doi: 10.1523/JNEUROSCI.0910-11.2011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su JD, Qiu J, Zhong YP, Li XY, Wang JW, Chen YZ. Expression of estrogen receptor (ER)-alpha and -beta immunoreactivity in hippocampal cell cultures with special attention to GABAergic neurons. J Neurosci Res. 2001;65:396–402. doi: 10.1002/jnr.1166. [DOI] [PubMed] [Google Scholar]
- Sui L, Wang J, Li BM. Role of the phosphoinositide 3-kinase-Akt- mammalian target of the rapamycin signaling pathway in long-term potentiation and trace fear conditioning memory in rat medial prefrontal cortex. Learn Mem. 2008;15:762–776. doi: 10.1101/lm.1067808. [DOI] [PubMed] [Google Scholar]
- Szymczak S, Kalita K, Jaworski J, Mioduszewska B, Savonenko A, Markowska A, Merchenthaler I, Kaczmarek L. Increased estrogen receptor beta expression correlates with decreased spine formation in the rat hippocampus. Hippocampus. 2006;16:453–463. doi: 10.1002/hipo.20172. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Horiike Y, Matsuzaki M, Miyazaki T, Ellis-Davies GC, Kasai H. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science. 2008;319:1683–1687. doi: 10.1126/science.1152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Reveron A, Khalid S, Williams TJ, Waters EM, Jacome L, Luine VN, Drake CT, McEwen BS, Milner TA. Hippocampal dynorphin immunoreactivity increases in response to gonadal steroids and is positioned for direct modulation by ovarian steroid receptors. Neuroscience. 2009;159:204–216. doi: 10.1016/j.neuroscience.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan N, Kow LM, Pfaff DW. Early membrane estrogenic effects required for full expression of slower genomic actions in a nerve cell line. Proc Natl Acad Sci U S A. 2001;98:12267–12271. doi: 10.1073/pnas.221449798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW. Membrane-initiated actions of estrogens in neuroendocrinology: emerging principles. Endocr Rev. 2007;28:1–19. doi: 10.1210/er.2005-0021. [DOI] [PubMed] [Google Scholar]
- Walf AA, Koonce C, Manley K, Frye CA. Proestrous compared to diestrous wildtype, but not estrogen receptor beta knockout, mice have better performance in the spontaneous alternation and object recognition tasks and reduced anxiety-like behavior in the elevated plus and mirror maze. Behav Brain Res. 2009;196:254–260. doi: 10.1016/j.bbr.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M, Luine V, Arellanos A, Frankfurt M. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res. 2006 doi: 10.1016/j.brainres.2006.07.064. [DOI] [PubMed] [Google Scholar]
- Walmer DK, Wrona MA, Hughes CL, Nelson KG. Lactoferrin expression in the mouse reproductive tract during the natural estrous cycle: correlation with circulating estradiol and progesterone. Endocrinology. 1992;131:1458–1466. doi: 10.1210/endo.131.3.1505477. [DOI] [PubMed] [Google Scholar]
- Warren SG, Humphreys AG, Juraska JM, Greenough WT. LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain Res. 1995;703:26–30. doi: 10.1016/0006-8993(95)01059-9. [DOI] [PubMed] [Google Scholar]
- Warren SG, Juraska JM. Spatial and nonspatial learning across the rat estrous cycle. Behav Neurosci. 1997;111:259–266. doi: 10.1037//0735-7044.111.2.259. [DOI] [PubMed] [Google Scholar]
- Waters EM, Mitterling K, Spencer JL, Mazid S, McEwen BS, Milner TA. Estrogen receptor alpha and beta specific agonists regulate expression of synaptic proteins in rat hippocampus. Brain Res. 2009;1290:1–11. doi: 10.1016/j.brainres.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters EM, Yildirim M, Janssen WG, Lou WY, McEwen BS, Morrison JH, Milner TA. Estrogen and aging affect the synaptic distribution of estrogen receptor beta-immunoreactivity in the CA1 region of female rat hippocampus. Brain Res. 2011;1379:86–97. doi: 10.1016/j.brainres.2010.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland NG, Orikasa C, Hayashi S, McEwen BS. Distribution and hormone regulation of estrogen receptor immunoreactive cells in the hippocampus of male and female rats. J Comp Neurol. 1997;388:603–612. doi: 10.1002/(sici)1096-9861(19971201)388:4<603::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Zhang Z. Effects of estradiol benzoate on learning-memory behavior and synaptic structure in ovariectomized mice. Life Sci. 2006;79:1553–1560. doi: 10.1016/j.lfs.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Yang PC, Yang CH, Huang CC, Hsu KS. Phosphatidylinositol 3- kinase activation is required for stress protocol-induced modification of hippocampal synaptic plasticity. J Biol Chem. 2008;283:2631–2643. doi: 10.1074/jbc.M706954200. [DOI] [PubMed] [Google Scholar]
- Yildirim M, Janssen WG, Tabori NE, Adams MM, Yuen GS, Akama KT, McEwen BS, Milner TA, Morrison JH. Estrogen and aging affect synaptic distribution of phosphorylated LIM kinase (pLIMK) in CA1 region of female rat hippocampus. Neuroscience. 2008;152:360–370. doi: 10.1016/j.neuroscience.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers KA, O'Brien PM, Eriksson E. Premenstrual syndrome. Lancet. 2008;371:1200–1210. doi: 10.1016/S0140-6736(08)60527-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen G. Laboratory of Neuroendocrinology), vol Ph.D. New York: The Rockefeller University; 2006. Estrogen action on actin dynamics: Mechanism for Dendritic Spine Remodeling in the Adult Hippocampus; p. 138. [Google Scholar]
- Yuen GS, McEwen BS, Akama KT. LIM kinase mediates estrogen action on the actin depolymerization factor Cofilin. Brain Res. 2011;1379:44–52. doi: 10.1016/j.brainres.2010.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Brinton RD. Select estrogens within the complex formulation of conjugated equine estrogens (Premarin) are protective against neurodegenerative insults: implications for a composition of estrogen therapy to promote neuronal function and prevent Alzheimer's disease. BMC Neurosci. 2006;7:24. doi: 10.1186/1471-2202-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Koldzic-Zivanovic N, Clarke CH, de Beun R, Wassermann K, Bury PS, Cunningham KA, Thomas ML. Selective estrogen receptor modulator effects in the rat brain. Neuroendocrinology. 2002;75:24–33. doi: 10.1159/000048218. [DOI] [PubMed] [Google Scholar]
- Znamensky V, Akama KT, McEwen BS, Milner TA. Estrogen levels regulate the subcellular distribution of phosphorylated Akt in hippocampal CA1 dendrites. J Neurosci. 2003;23:2340–2347. doi: 10.1523/JNEUROSCI.23-06-02340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Korol DL. Estrogen modulates place learning through estrogen receptors in the hippocampus. Neurobiol Learn Mem. 2006;86:336–343. doi: 10.1016/j.nlm.2006.07.008. [DOI] [PubMed] [Google Scholar]