SUMMARY
A general mechanism for how intracellular signaling pathways in human pluripotent cells are co-ordinated and how they maintain self-renewal remain to be elucidated. In this report, we describe a novel signaling mechanism where PI3K/Akt activity maintains self-renewal by restraining pro-differentiation signaling through suppression of the Raf/Mek/Erk and canonical Wnt signaling pathways. When active, PI3K/Akt establishes conditions where Activin A/Smad2,3 performs a pro-self renewal function by activating target genes, including Nanog. When PI3K/Akt signaling is low, Wnt effectors are activated and function in conjunction with Smad2,3 to promote differentiation. The switch in Smad2,3 activity following inactivation of PI3K/Akt requires the activation of canonical Wnt signaling by Erk, which targets Gsk3β. In sum, we define a signaling framework that converges on Smad2,3 and determines its ability to regulate the balance between alternative cell states. This signaling paradigm has far-reaching implications for cell fate decisions during early embryonic development.
INTRODUCTION
Pluripotent stem cells are maintained in a stable, self-renewing state under conditions where cell signaling pathways block differentiation and support long-term cell division. A fundamental understanding of how signaling pathways underpin pluripotency has not been reached however, and is complicated by the use of disparate culture conditions to address this question. Despite this, several signaling pathways are known to impact significantly on the fate of pluripotent cells (reviewed by Ohtsuka and Dalton, 2008). These include signaling through insulin-like growth factor (Igf)/PI3K, Activin A/Smad2,3, Raf/Mek/Erk and Wnt/Gsk3β signaling pathways. While each of these pathways has a demonstrated role in some aspect of self-renewal the regulatory network underpinning pluripotency has not been elucidated.
TGF-β/Activin A signaling is essential for the self-renewal of human embryonic stem cells (hESCs; Beattie et al., 2005; Xiao et al., 2006) and functions by activating Smad2,3 via its binding to the Alk4/Activin Receptor Type II. Upon activation and dimerization, Smad2,3 maintains the pluripotent state through regulation of Nanog transcription (Xu et al., 2008; Vallier et al., 2009). Under some conditions, Activin A cooperates with Fgf2 signaling to support pluripotency (Vallier et al., 2005), although the molecular mechanism underpinning this interaction has not been defined in detail. Paradoxically, although Activin A has a clear role in self-renewal, it also has well-defined roles in promoting the initial differentiation events in the epiblast and in cell-fate commitment of embryonic stem cells (McLean et al., 2007). In developmental models such as Xenopus, Activin/Nodal signaling regulates the induction of mesendoderm genes such as XBRA (Xenopus Brachyury) and GSC (Goosecoid; Heasman, 2006) and is therefore required for the establishment of mesoderm and endoderm lineages (Wardle and Smith, 2006). How Activin A promotes self-renewal under one set of conditions and differentiation under others is not understood.
In murine ESCs (mESCs), Erk signaling antagonizes self-renewal and so for the pluripotent state to be maintained, Mek/Erk activity must be suppressed (Wray et al., 2010). Considerable confusion surrounds the role of Raf/Mek/Erk signaling in hESCs as indicated by a number of conflicting reports, some advocating roles in maintenance of pluripotency (Armstrong et al., 2006; Li et al., 2007), while other suggest it has roles in promoting differentiation (Ding et al., 2010; Na et al., 2010). In the embryo however, Erk signaling has well-defined roles in mesendoderm induction (Wardle and Smith, 2006), consistent with it antagonizing self-renewal signaling in mESCs.
The canonical Wnt pathway is associated with early cell fate decisions made by pluripotent cells during gastrulation. Its role in pluripotent cells in vitro however, has been difficult to decipher again because of conflicting reports. Some studies suggest roles for Wnt signaling and inhibition of Gsk3β in hESCs maintenance (Sato et al., 2004), while others support in vivo data indicative of roles in mesendoderm induction via way of an epithelial to mesenchymal transition (EMT; Sumi et al., 2008). Consistent with the latter, key effectors of canonical Wnt signaling such as β-catenin, directly regulate mesendoderm development through target genes such as XBRA (Heasman, 2006).
While Activin A/Smad2,3, Raf/Mek/Erk and Wnt/Gsk3β signaling have roles in embryonic lineage commitment of pluripotent cells, PI3K signaling is specifically associated with maintenance of pluripotency in vitro (McLean et al., 2007; Storm et al., 2007; Watanabe et al., 2006). PI3K maintains human pluripotent cells by suppressing the ability of specification factors, such as Activin A, to promote differentiation (McLean et al., 2007). This finding indicates that PI3K/Akt cooperates with Activin A to promote the pluripotent state and suggests that Activin A has context-dependent functions in promoting and antagonizing self-renewal pathways.
While several of the major intracellular signaling pathways impacting on the self-renewal of hESCs and human induced pluripotent stem cells (hiPSCs) have been identified, their mechanism of action and potential ability to cross-talk as part of an integrated network has not been addressed. In this report, we define a novel regulatory network that integrates these key pathways thereby explaining the basic principles of self-renewal from a cell signaling perspective. The regulatory network we define establishes the crosstalk between PI3K/Akt, Raf/Mek/Erk, and Wnt/Gsk3β which together impacts on the ability of Activin A/Smad2,3 to either promote self-renewal or differentiation. This model is consistent with developmentally established principles of early cell fate commitment and has broad-reaching implications not just for pluripotent stem cells in culture but also for understanding early fate decisions in the embryo.
RESULTS
PI3K governs the ability of Activin A to promote pluripotency or differentiation
Inhibition of PI3K/Akt signaling in hESCs cultured with MEF-CM is incompatible with maintenance of pluripotency and promotes differentiation to mesoderm and endoderm (McLean et al., 2007). The molecular mechanisms underpinning the role of signaling pathways in maintenance of pluripotent cells including PI3K are however, poorly understood. To broadly understand cell signaling networks in hESCs and how PI3K maintains pluripotency we utilized a defined culture system based on StemPro® hESC SFM (HAI; Wang et al., 2007). The rationale being that in a defined system factors can be added and subtracted, making it possible to evaluate their individual roles in cell signaling and maintenance of pluripotency. Only three recombinant factors are required for hESC self-renewal in HAI/StemPro® defined media; heregulin (H), Activin A (A) and Igf-1 (I). Heregulin and Igf-1 represent potent activators of PI3K/Akt1 signaling while Activin A is a regulator of pluripotency factors such as Nanog through its activation of Smad2,3 (Xu et al., 2008; Vallier et al., 2009). Fgf2 was not required in this media as it is functionally redundant with heregulin and, because it functions only at high concentrations (~100ng/ml; Levenstein et al., 2006) was omitted from many of our experiments. As is seen following inhibition of PI3K in hESCs maintained in MEF-CM, addition of the small molecule inhibitor LY 294002 to HAI defined media promoted the upregulation of mesendoderm transcript markers such as Brachyury, Eomes, Goosecoid (Gsc) and MixL1 (Supplementary Figure 1). This is a similar response observed when hESCs cultured in MEF-CM are treated with LY 294002 (McLean et al., 2007). Additionally, inhibitors of Akt (Akt inhibitor XI and API-59CJ-OMe) and shRNA knockdown of Akt1, 2, and 3 also promoted the up-regulation of mesendoderm transcript markers (Supplementary Figure 1)
Omission of Igf-1 and heregulin (−HI) from defined media had a similar effect to that of LY 294002 treatment. Most notably, mesendoderm marker transcripts Eomes and MixL1 increased within 2 days and pluripotency markers Nanog and Oct4 declined by day 4 (Figure 1A). Immunostaining revealed an increase in Brachyury protein at day 2 and a decrease in Nanog protein within 4 days (Figure 1B). Loss of Akt1 activity, as judged by its phosphosphorylation on residue threonine 308 and serine 473 (pAktT308 and pAktS473), and ribosomal S6 phosphorylation decreased within 24 hours (Figure 1C). These results indicate that Igf-1 and heregulin are activators of Akt in hESCs. In addition to the data shown using BG02 hESCs, these general findings were replicated in WA01, WA07 and WA09 hESC lines (Supplementary Figure 1). To formally show that Akt is a major effector of PI3K signaling in hESCs a myristoylated, constitutively active version of Akt (myr.AKT) was expressed in cells cultured in the absence of Igf-1 and heregulin. This blocked the up-regulation of Eomes, Gsc and MixL1 transcripts following loss of PI3K activity (Figure 1D), showing that Akt1 is a major effector of the PI3K-dependent differentiation blockade in hESCs. Furthermore, a stable hESC line expressing myr.AKT was able to maintain pluripotency markers in the absence of heregulin and Igf-1 for more than 5 passages (Supplementary Figure 1). These data indicate that Akt is the major effector of heregulin and Igf-1 signaling, and is required to maintain pluripotency.
Figure 1. Activin A promotes self-renewal or differentiation depending on the status of PI3K/AKT signaling.
(A) Transcript markers of BG02 hESCs in −HI. (B) Immunostaining of BG02 hESCs in −HI. Micron bar, 50 μm. (C) Immunoblot analysis of BG02 hESC lysates in HAI or −HI for 24 hours. (D) Transcript markers after transfection of WA09 hESCs with a myr.AKT-IRES-GFP expression vector in +HAI media. 24 hours after transfection GFP+ and GFP− cells were isolated by FACS, plated and then grown in −HI for 3 days. (E) Transcript markers in −HI in WA09 hESCs after 4 days +/− SB 431542 (20 μM). (F) Immunoblot analysis of BG02 hESC lysates from cells grown in −HI for 2 and 4 days, +/− SB 431542. (G) MixL1 promoter-luciferase assays. 24 hours after transfection, WA09 hESCs were cultured for 2 days in +HAI, −HI or −HI +SB 431542 and assayed. (H) Immunoblot analysis of BG02 hESC in +HAI media and starved of factors for 18 hours. Factors were re-added for 3 hours as indicated. H, heregulin (10 ng/ml); A, Activin A (10 or 100 ng/ml); I, Igf-1 (200 ng/ml); F10, Fgf2 (10 ng/ml). (I) Model summarizing results from Figure 1. *P<0.05, **P<0.01. See also Supplementary Figure 1.
Another major self-renewal pathway identified in hESCs depends on the signaling of Activin A through Smad2,3 (Beattie et al., 2005; Xiao et al., 2006). On the surface this seems counterintuitive since Activin A and Smad2,3 are known inducers of differentiation and yet in hESCs they also perform a role in hESC maintenance. To evaluate the potential dual role of Activin A in self-renewal and differentiation we used SB 431542 (SB), an antagonist of TGFβ receptors (Alk4,5,7). Addition of SB blocked the up-regulation of differentiation markers in response to withdrawal of PI3K/Akt activators Igf-1 and heregulin (Figure 1E,F), indicating that Activin A/Smad2,3 signaling is required for mesendoderm induction. Nanog transcript and protein levels also declined following treatment with SB under self-renewal (+HAI) and differentiation (−HI) conditions (Figure 1F and data not shown). This is consistent with established roles for Activin A/Smad2,3 signaling in regulation of Nanog transcription (Xu et al., 2008; Vallier et al., 2009). Together, these results indicate that Activin A/Smad2,3 promotes self-renewal when PI3K/Akt activity is elevated but promotes differentiation when PI3K/Akt activity is low.
To investigate the relationship between PI3K/Akt and Activin A further, we focused on transcriptional regulation of MIXL1, a mesendoderm gene that is rapidly activated following loss of PI3K/Akt signaling. Using a MixL1-luciferase reporter, we found that loss of PI3K signaling (−HI) increased luciferase activity and that this was blocked by addition of SB (Figure 1G). Loss of PI3K/Akt signaling was a requirement for the activation of the reporter even in increasing concentrations of Activin A (Supplementary Figure 1). The ability of Activin A to transcriptionally activate genes associated with mesendoderm induction therefore requires conditions where PI3K activity is low.
Towards understanding how PI3K controls cell fate through Activin A signaling we asked if Smad2,3 activity varies in response to changes in PI3K/Akt activity. In this scenario, a threshold of Smad2,3 activity would control a switch that determines the decision to self-renew or differentiate. This was addressed by assessing Smad2,3 phosphorylation under conditions where PI3K/Akt is active or inactive (+/− HI). Under self-renewing conditions (+HAI) a baseline of activated Smad2,3 was detected, consistent with its requirement for self-renewal (Figure 1H). In the absence of PI3K activation, Akt1 activity was lost and surprisingly Smad2,3 activity was significantly elevated. Smad2,3 activity and Nanog levels under these conditions were dependent on Activin A in a dose-dependent manner. This trend was reversed however when Akt1 signaling was restored by addition of HI or HIF (Figure 1H). Increased activity of Smad2,3 in the absence of PI3K signaling (−HI) was confirmed using an Activin response element (ARE)-luciferase reporter construct (Supplementary Figure 1; Weisberg et al., 1998). ARE activation is dependent on Smad2,3 but independent of Wnt, as shown by insensitivity to Dkk1. The status of PI3K/Akt signaling therefore regulates the threshold levels of Activin A/Smad2,3 signaling to activate different subsets of target genes to impact on cell-fate decisions. A model to explain these findings is shown in Figure 1I.
The PI3K self-renewal signal suppresses Erk and maintains Gsk3β activity
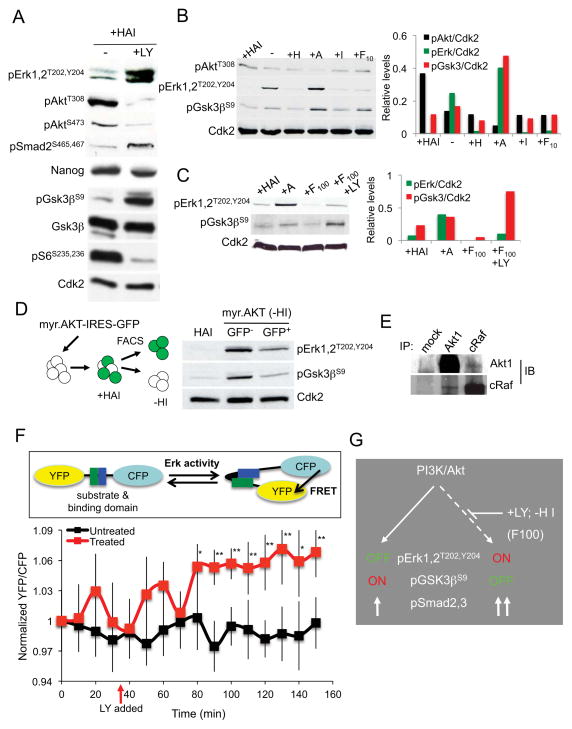
Upon further examination of signaling pathways in response to PI3K inhibition, we observed that phosphorylation of Erk1,2 (pErk1,2T202,Y204) and Gsk3β (pGsk3βS9; Figure 2A) increased within 12 hours of LY 294002 treatment. These changes represent activation of the MAPK/Erk pathway and inactivation of Gsk3β activity, respectively. These data were also confirmed using MEF-CM (Supplementary Figure 2), indicating that the cell signaling networks downstream of PI3K are preserved in defined media and MEF-CM. Consistent with previous data (Figure 1), levels of activated Smad2,3 increased with loss of PI3K activity (Figure 2A). All of these signaling changes preceded loss of Nanog and are therefore early responses to self-renewal pathways being disrupted.
Figure 2. Activin A regulates Erk and Gsk3β in the absence of PI3K/AKT activity.
(A) Immunoblot of BG02 hESCs +/− LY 294002 (50 μM) for 12 hours. (B) WA09 hESCs were starved of factors for 24 hours (−HAI) then stimulated with factors as indicated for 3 hours; +H, heregulin (10 ng/ml); +A, Activin A (10 ng/ml); +I, Igf-1 (200 ng/ml); +F10, Fgf2 (10 ng/ml). (C) WA09 hESCs (+HAI) were treated and analyzed as in (B) with indicated factors for 3 hours. (D) WA09 hESCs (+HAI) were transfected with myr.AKT-IRES-GFP expression vector. GFP+ and GFP− cells were isolated by FACS, plated in +HAI media for 24 hours then plated in −HI media for 2 days. (E) Immunoprecipitation of WA09 hESCs (+HAI) with Akt1 and Raf1, followed by immunoblotting. (F) ERK activation measured as the ratio of YFP/CFP fluorescence in WA09 hESCs (+HAI) transfected with EKAR. The ratio average ± SEM for untreated cells (10; black line) and LY 294002-treated cells (7; red line). (G) Model summarizing data presented in the Figure. *P<0.05, **P<0.01. See also Supplementary Figure 2.
To understand more precisely the signaling requirements controlling Erk1,2 and Gsk3β activity in hESCs, cells were starved of all factors for 24 hours (−HAI), then stimulated with the indicated factors for a further 3 hours. In the absence of all three factors (low Akt1 activity), Erk1,2 was activated and Gsk3β inactivated (Figure 2B), consistent with responses seen in Figure 2A when PI3K is directly inhibited with LY 294002. Igf-1 and heregulin blocked these changes whereas Activin A did not, consistent with PI3K activation being required for these events. Heregulin and Igf-1 displayed a synergistic effect in their ability to activate Akt (Supplementary Figure 2), explaining the requirement for both factors in StemPro®/HAI media. At low levels Fgf2 (F10; 10ng/ml) had only a minimal effect on Erk1,2 and Gsk3β, but when increased to 100ng/ml (F100) it reproduced the effects seen with addition of Igf-1 and heregulin (Figure 2C), indicating that it operates through a similar signaling cascade to these factors. This is consistent with Fgf2 being able to substitute for heregulin in long-term self-renewal assays when present at high levels (data not shown; see Discussion).
To directly address the role of Akt1 in regulation of Erk1,2 and Gsk3β, WA09 hESCs were transfected with a myr.AKT-IRES-GFP expression construct in complete defined media (+HAI). Following sorting, GFP+/− cells were grown in −HI media and assayed by immunoblot analysis to evaluate the status of Erk1,2 and Gsk3β (Figure 2D). Cells expressing myr.AKT1 had low Erk1,2 activity and elevated Gsk3β activity in contrast to GFP− cells, consistent with results shown in Figure 2A. Maintaining Akt1 activity in pluripotent cells is therefore critical for suppression of Erk1,2 activity and for the maintenance of Gsk3β activity.
Previous studies have demonstrated that Akt can directly bind and phosphorylate cRaf, leading to the inhibition of Raf/Mek/Erk signaling (Rommel et al., 1999). Immunoprecipitation assays performed in hESCs confirmed an Akt1-cRaf interaction suggesting that this is the mechanism by which Akt blocks Erk signaling (Figure 2E).
To further examine the ability of Akt to repress Erk signaling, we utilized Fluorescence Resonance Energy Transfer (FRET) and the EKAR/Erk FRET reporter (Harvey et al., 2008). Following transfection of hESCs with EKAR, PI3K inhibition using LY 294002 led to a strong FRET response after approximately 45 min (Figure 2F). These data demonstrate that PI3K/Akt inhibits Erk activity in vivo. In total, these data indicate that the PI3K pathway cross-talks with Erk1,2 and Gsk3β signaling pathways to maintain hESCs (Figure 2G).
Raf/Mek/Erk antagonizes self-renewal by inhibition of Gsk3β
Although Erk signaling increases under conditions of low PI3K/Akt activity, the significance of this in terms of self-renewal is unclear. Roles for elevated Erk activity were therefore evaluated by overexpression of a dominant-negative Erk1 (dnErk1) or by addition of the Mek inhibitor, UO126. In both cases the up-regulation of early differentiation markers such as Brachyury, Eomes, Gsc and MixL1 was blocked following loss of PI3K signaling (−HI) and Erk suppression (Figure 3A–C). UO126 also blocked the down-regulation of Nanog under these conditions (Figure 3C). Not only did Mek inhibition block differentiation in low PI3K/Akt activity, it also suppressed the inactivation of Gsk3β (Figure 3D), supporting the idea that Mek/Erk signaling inhibits Gsk3β activity.
Figure 3. Raf/Mek/Erk signaling promotes the differentiation of hESCs and inhibits Gsk3β.
(A) Transcript markers in −HI for 3 days, with or without transfection of dnErk1 from WA09 hESCs. (B) Transcript levels from WA09 hESCs (−HI) after 4 days +/− U0126 (20 μM). (C) Immunoblot analysis of BG02 hESC lysates (−HI) for 2 and 4 days, +/− U0126. (D) Immunoblot analysis of WA09 hESC lysates in −HI, +/− U0126. (E) Transcript markers after transfection and FACS of WA09 hESCs with a constitutively active-cRAF-IRES-GFP vector after culture (+HAI) for 3 days. (F) Immunoblot analysis after transfection and FACS of WA09 hESCs with a constitutively-active cRAF-IRES-GFP vector. Cell lysates prepared after a further 3 hours culture in −HI media. (G) Immunoblot analysis of WA09 hESC lysates (+HAI) having a constitutively-active MEK-ER transgene or empty vector, +/− 100 nM 4-hydroxy-tamoxifen (4OHT) for 24 hours. (H) Transcript markers of WA09 hESCs containing a constitutively active MEK-ER transgene or vector control, +/− 100 nM of 4OHT for 3 days (+HAI). (I) Immunoprecipitation of WA09 hESCs (+HAI) with Erk1,2 and Gsk3β, followed by immunoblotting. (J) Immunoprecipitation (IP)-kinase assays for Gsk3β, of WA09 hESCs cultured in media with +HAI, −HI, −HI +U0126, or −HI +Dkk1 (150 ng/ml). (K) Luciferase assay of WA09 hESCs treated LY 294002 +/− U0126 for 2 days after transfection of Top-Flash or Fop-Flash reporters. (L) Model summarizing the data presented in Figure 3. *P<0.05, **P<0.01.
The role of Raf, an upstream regulator of Mek and Erk, was then evaluated by ectopic expression of a constitutively active cRaf mutant (McCubrey et al., 1998). Cells expressing cRaf (GFP+) up-regulated differentiation markers even under conditions where PI3K/Akt remained active (Figure 3E). Cells not expressing active cRaf (GFP−) maintained low levels of Eomes, Gsc and MixL1 mRNAs. Immunoblot analysis shows that cRaf activated Erk1,2 and inactivated Gsk3β (Figure 3F). Expression of tamoxifen-regulated, constitutively active Mek (MEK-ER; Blalock et al., 2000), had similar effects on Erk1,2 and Gsk3β activity, and mesendoderm differentiation markers as was seen for activated cRaf (Figure 3G, 3H). Previous reports have found that Erk directly binds Gsk3β, which promotes its inactivation through a priming phosphorylation (Ding et al., 2005). Immunoprecipitation (IP) assays confirmed that this interaction also occurs in hESCs (Figure 3I) indicating that Erk regulates Gsk3β directly. Next, the requirement for Mek activity in Gsk3β inactivation was evaluated using IP-kinase assays. These assays show that under conditions of low PI3K activity (−HI), UO126 blocks the inactivation of Gsk3β kinase (Figure 3J). As β-catenin is a direct effector of Wnt/Gsk3β signaling, we asked if it was activated following loss of PI3K activity, using the Top-Flash/Fop-flash luciferase reporter and LY 294002. Top-Flash activity increased following treatment with LY 294002, but was blocked by addition of the Mek inhibitor, UO126. These results show that the activation of Erk1,2 inactivates Gskβ, in response to reduced PI3K signaling. Following this, Wnt effectors such as β-catenin are activated (see Figure 3L).
GSK3β is required for the self-renewal of human pluripotent stem cells
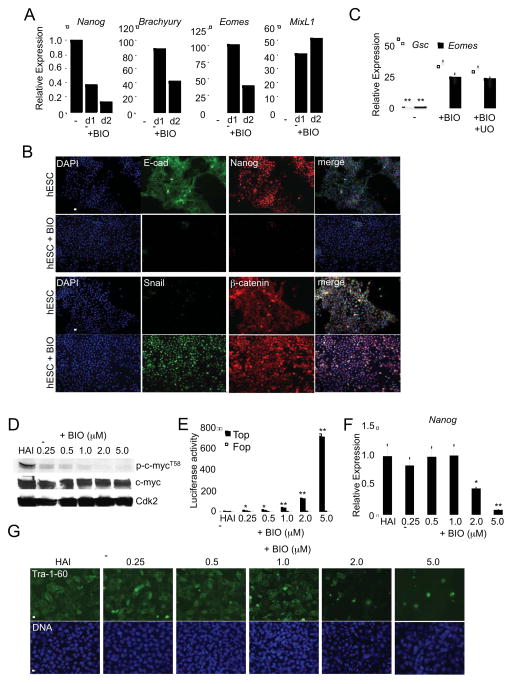
By several criteria we have shown that Gsk3β inactivation, following the loss of PI3K/Akt signaling and activation of Erk1,2, coincides with loss of self-renewal. This suggests that Gsk3β is required to maintain pluripotent cells in a self-renewing state. To investigate this, we treated hESCs with a Gsk3β small molecule inhibitor (BIO) and show that it promotes the down-regulation of Nanog and up-regulation of Brachyury, Eomes and MixL1 transcripts over a period of 1–2 days (Figure 4A). This coincided with loss of E-cadherin and Nanog protein in addition to the nuclear accumulation of β-catenin and Snail (Figure 4B). Together, these events are characteristic of an EMT and indicate that Gsk3β inhibition drives pluripotent cells into an early-differentiated state by activation of canonical Wnt signaling effectors. These findings were reproduced with hESCs grown in MEF-CM, mTeSR1 and with other Gsk3β inhibitors (Supplementary Figures 3 and 4).
Figure 4. Loss of Gsk3β activity promotes mesendoderm differentiation of hESCs.
(A) Transcript markers in BG02 hESCs in +HAI media, +/− BIO (2 μM). (B) Immunofluorescence of BG02 hESCs +/− BIO. Micron bar, 100 μm. (C) Transcript markers of WA09 hESCs with BIO +/− U0126 for 3 days. (D) Immunoblot analysis of WA09 hESCs treated with increasing doses of BIO after 24 hours. (E) Luciferase assays in WA09 hESCs treated with increasing doses of BIO, 48 hours after transfection. (F) Transcript markers of WA09 hESCs treated with increasing doses of BIO for 72 hours. (G) Immunofluorescence of WA09 hESCs treated with increasing doses of BIO for 72 hours. *P<0.05, **P<0.01. (F). *P<0.05, **P<0.01. See also Supplementary Figures 3 and 4.
To demonstrate that Gsk3β is required for hESC self-renewal using a genetic approach we assayed the effects of a Cre-activated, dominant-negative (dn) version of Gsk3β (Supplementary Figure 4C; Hagen et al., 2002). Following Cre expression, elevated levels of Brachyury and Snail were seen in the nucleus while levels of Nanog, E-cadherin and β-catenin declined (Supplementary Figure 4D). Interfering with Gsk3β activity therefore activates events reminiscent of an EMT and events associated with mesendoderm induction. To establish if Gsk3β lies downstream of Mek/Erk we asked if UO126 could block the up-regulation of differentiation markers following BIO treatment. By itself, Gsk3β inhibition with BIO up-regulates differentiation markers but is unaffected by Mek inhibition (Figure 4C). Gsk3β inactivation is therefore sufficient to promote differentiation, indicating that a major function of Erk is to regulate Gsk3β. These data are consistent with known roles for Gsk3β as an antagonist of canonical Wnt signaling and provide a model for the basic signaling requirements that promote self-renewal.
In contrast to our observations, inhibition of Gsk3 has been reported to promote self-renewal of hESCs in short-term assays (Sato et al., 2004). Other recent studies however, show that Gsk3 inhibitors have dose-dependent effects where at low concentrations they promote self-renewal but at higher concentrations they promote differentiation (Tsutsui et al., 2011; Li et al., 2011). One explanation for these phenomena is that different pools of Gsk3 in the cell have different activation thresholds for signaling. For example, Gsk3 is a target of PI3K/Akt signaling in addition to being a regulator of the canonical Wnt pathway- these compartments are physically separate and regulated by distinct mechanisms (Voskas et al. 2010). This establishes a potential scenario where different doses of Gsk3 inhibitors may result in differing biological outcomes. To test this hypothesis, hESCs were treated with increasing concentrations of the Gsk3 inhibitor, BIO. Interestingly, we found that BIO treatment at low concentrations (<1 μM) led to decreased phosphorylation on c-myc on threonine-58 (Figure 4D). Decreased T58 phosphorylation, leading to enhanced c-myc stability, is known to be a positive determinant of self-renewal in ESCs (Cartwright et al., 2005). Gsk3 inhibition under these conditions however, had a negligible effect on β-catenin Top-Flash reporter activity indicating that canonical Wnt signaling was not affected under these conditions (Figure 4E). Importantly, loss of pluripotency markers such as Nanog and Tra-1–80, only occurred at higher concentrations of BIO (>2.0 μM), coinciding with activation of the Top-Flash reporter (Figure 4F and 4G). These data show that lower concentrations of Gsk3 inhibitor promotes self-renewal through mechanisms such as Myc stabilization, while higher concentrations of Gsk3 inhibitor promotes differentiation through activation of Wnt/β-catenin. In summary, these results indicate the existence of a cross-talk mechanism between PI3K/Akt, Raf/Mek/Erk and Gsk3β signaling that controls self-renewal in human pluripotent cells.
Wnt signaling antagonizes self-renewal in human pluripotent cells
Although elevated Wnt/β-catenin signaling promotes differentiation, basal β-catenin activity under self-renewing conditions may be important for sustaining pluripotency (Sumi et al., 2008). Although exogenous Wnt ligands are not included in our culture media, the low level of basal Wnt activity detected in our assays is most likely due to an autocrine production by hESCs themselves (Supplementary Figure 5). To investigate possible roles for basal Wnt/β-catenin activity in self-renewal, we inhibited Wnt activity using Dkk1. Under these conditions, hESCs could be maintained for >1 month without any adverse effects on cellular morphology or loss of pluripotency markers (Figure 5A and data not shown). Inhibition of Wnt signaling by Dkk1 was confirmed using the Top-Flash/Fop-Flash reporter assay (Figure 5B). Since a background level of differentiation is typically seen in most hESC cultures we asked if this could be suppressed by inhibition of Wnt signaling using Dkk1. Addition of Dkk1 almost completely suppressed the small percentage of Brachyury positive cells routinely seen in hESC cultures while retaining Nanog expression (Figure 5A). As a more sensitive assay using qRT-PCR, we showed that background levels of Brachyury, Eomes, Gsc, Sox17 and Gata6 transcripts are suppressed in cultures maintained in the presence of Dkk1, while Nanog transcripts increased slightly (Figure 5C). By flow cytometry, the percentage of SSEA3+ cells increased in the presence of Dkk1 (Figure 5D). Taken together, these data indicate that suppression of Wnt signaling stabilizes pluripotent cells.
Figure 5. Inhibition of Wnt signaling by Dkk1 stabilizes human pluripotent cells.
(A) Immunofluorescence analysis of WA09 hESCs in +HAI, +/− Dkk1 (150 ng/ml) for 6 passages. Micron bar, 50 μm. (B) Luciferase assay of WA09 hESCs in +HAI, +/− Dkk1 for 6 passages 2 days after transfection. Values expressed as ratio of Top-Flash to Fop-Flash signals. (C) Transcript levels in WA09 hESCs (+HAI) after treatment with Dkk1 for 4 days. (D) Flow cytometry analysis of WA09 hESCs cultured +/− Dkk1 for 6 passages. (E) Transcript markers in −HI, +/− Dkk1 (F) Transcript markers after transfection and FACS of a constitutively active cRAF-IRES-GFP vector in +HAI, +/− Dkk1 for 3 days. (G) Model summarizing the data from Figures 4 and 5. *P<0.05, **P<0.01. See also Supplementary Figure 5.
Our data shows that Erk-dependent inactivation of Gsk3β signaling has a major role in destabilizing the pluripotent state. Next, we asked if the canonical Wnt pathway was a focal point for this regulation by asking if Wnt ligands generate a signal that destabilizes pluripotent cells under conditions of low PI3K activity (−HI) and elevated Raf/Mek/Erk activity. This was first addressed by culturing hESCs in the absence of HI and in the presence or absence of Dkk1. Inhibition of Wnt signaling by Dkk1 blocked the activation of early differentiation markers in this assay (Figure 5E), confirming that destabilization of hESCs following loss of PI3K/Akt is dependent on Wnt signaling. Dkk1 did not have any non-specific effects on the phosphorylation status of Erk, Gsk3β, Smad2 or c-myc consistent with its function of specifically blocking Wnt signaling (Supplementary Figure 5). Dkk1 also blocked the up-regulation of differentiation markers following the expression of active cRaf (Figure 5F). Both of these findings are consistent with our model that a cross-talk mechanism between PI3K, Erk and Gsk3β converges on Wnt signaling (Figure 5G).
Smad and Wnt signaling pathways coordinately converge on target genes
A key finding of our work so far shows that Activin A/Smad2,3 switches from having a pro-self renewal function to a pro-differentiation function in the absence of PI3K/Akt signaling. A mechanism underpinning these observations is suggested from our findings that Activin A/Smad2,3 promotes differentiation through activation of Erk signaling, through the subsequent repression of Gsk3β and following activation of Wnt effectors such as β-catenin and Snail. Since Activin A/Nodal signaling induces mesoderm and definitive endoderm development in the vertebrate embryo, we asked if Smad2,3 signaling synergizes with the Wnt pathway to promote the activation of mesendoderm genes in hESCs. This would therefore test whether the combinatorial action of Activin A with Wnt effectors switches the activity of Smad2,3 to a pro-differentiation function. To test this hypothesis, mesendoderm differentiation was induced by the Gsk3 inhibitor/Wnt pathway agonist BIO, in the presence or absence of SB 431542 (SB; Figure 6A). As expected, Gsk3 inhibition up-regulated the mesendoderm markers Eomes, Gsc and MixL1 but this was blocked by inhibiting Activin A signaling with SB. Nanog was down-regulated in BIO, or in BIO and SB, consistent with our findings that Gsk3 inhibition promotes the loss of pluripotency and the role of Activin A/Smad2,3 in regulating Nanog (Figures 1E and 1F; Xu et al., 2008; Vallier et al., 2009). Additionally, we have recently described the use of BIO and SB as a method to promote neural crest differentiation from hESCs (Menendez et al., 2011). This indicates that Wnt effectors (ie. β-catenin) act in conjunction with Smad2,3 to activate mesendoderm genes. SB also blocked the BIO-dependent activation of a MixL1-luciferase reporter, indicating that this regulation occurs at the level of transcription (Figure 6B). To evaluate the binding of Smad2,3 and β-catenin during mesendoderm induction of hESCs, chromatin immunoprecipitation assays were performed (Figure 6C). Low levels of Smad2,3 were found on the MixL1 promoter in hESCs and following treatment with BIO. β-catenin binding however, increased significantly following BIO treatment. Interestingly, in the presence of SB, β-catenin binding to the MixL1 promoter was lost suggesting that the presence of Smad2,3 on the promoter is a prerequisite for binding by β-catenin. No significant changes of Smad2,3 enrichment on the Nanog promoter were observed in the presence of BIO (data not shown). These data show that upon Gsk3 inhibition, β-catenin binding at the MixL1 promoter increases indicating that it synergizes with Smad2,3 to promote transcription.
Figure 6. Wnt/β-catenin and Activin/Smad signaling cooperate to regulate mesendoderm gene expression.
(A) Transcript markers of WA09 hESCS grown in +HAI, +/− BIO (2 μM) +/− SB 431542 (20 μM). (B) MixL1-luciferase reporter assays in WA09 hESCs cultured in +HAI medium after addition of BIO +/− SB 431542 after 3 days. (C) Chromatin Immunoprecipitation assay of WA09 hESCs (+HAI) after the addition of BIO or BIO + SB 431542 for 12 hours with Smad2,3 and β-catenin on the MixL1 promoter. (D) MixL1-luciferase assays in +HAI media co-transfected with vector alone or caSmad3, +/− Dkk1 (150 ng/ml) for 3 days. (E) Transcript markers of WA09 hESCs following transfection and FACS for a caSmad3-IRES-GFP vector +/− Dkk1. *P<0.05, **P<0.01.
To further test the hypothesis that Smad and Wnt effectors cooperate to regulate mesendoderm markers, we transiently transfected hESCs (+HAI complete defined media) with constitutively active Smad3 (caSmad3; Funaba and Mathews, 2000), along with the MixL1-luciferase reporter (Figure 6D). Transfection of caSmad3 increased MixL1 promoter activity ~8- fold, supporting the idea that a threshold of Smad2,3 signaling in hESCs is limiting for activation of mesendoderm genes (Figure 7C). Since a low level of Dkk1-sensitive Wnt signaling can be detected in hESCs (see Figure 6) we included Dkk1 to establish if Wnt is also required for MixL1 transcription under these conditions. This was confirmed by the observation that in the presence of caSmad3, Dkk1 reduces MixL1 promoter activity to that below steady-state levels in control hESCs. Activation of MixL1 therefore requires Smad2,3 activity in combination with effectors of the canonical Wnt pathway. Furthermore the transfection of a caSmad3 up-regulated mesendoderm transcripts, but not in the presence of Dkk1 (Figure 6E), further demonstrating that Smad2,3 and β-catenin cooperate to activate mesendoderm genes. These data support the model that once PI3K/Akt activity declines, Activin A switches to a pro-differentiation mode of regulation by collaborating with the Wnt pathway. A model to describe the observations made throughout this report is shown in Figure 7A,B.
Figure 7. Model of signaling networks that regulate self-renewal and differentiation of human pluripotent stem cells.
(A) Model summarizing the self-renewing state, when PI3K/Akt signaling is active. Akt first modulates the threshold of Smad2,3 activity and second, inhibits Erk and maintains Gsk3β activity, compatible with Nanog expression. (B) Model summarizing the differentiated state. Upon PI3K/Akt inactivation, Activin A/Smad2,3 signaling is enhanced, Erk is activated and when coupled with Wnt signaling, promotes Gsk3β inhibition and β-catenin activation. Subsequently, Smad2/3 and Wnt effectors co-operate to promote the activation of mesendoderm markers, such as MixL1. Nanog expression is lost after 4 days of differentiation.
DISCUSSION
PI3K/Akt, Raf/Mek/Erk, Activin/Smad and Wnt/β-catenin signaling pathways have all been implicated in regulating human stem cell pluripotency. However, in a broader context, how these pathways cooperate to maintain the balance between self-renewal and differentiation has been unclear. Using a simple, defined culture system which forms the basis of StemPro® hESC SFM (Wang et al., 2007), we describe a novel cross-talk mechanism where PI3K/Akt suppresses the activation of pro-differentiation pathways centering around Raf/Mek/Erk and canonical Wnt signaling. The signaling cross-talk described here is widely applicable to all hESC media types, including MEF-CM, StemPro® and mTesr1®. Only two extrinsically-activated signaling pathways are required to maintain pluripotency. First, activation of Smad2,3 and its downstream targets, such as Nanog, by Activin A/Nodal (Xu et al., 2008; Vallier et al., 2009) and second, the activation of PI3K/Akt signaling by factors such as Igf-1, heregulin and Fgf2. These two signaling requirements can be identified in all hESC media formulations described to date. For example, serum contains high levels of Igfs and knockout serum replacement (KSR) contains high levels of insulin. Sources of Activin A include MEF feeder layers, MEF-CM in addition to its inclusion in defined media formulations (Ludwig et al., 2006; Wang et al., 2007; Yao et al., 2006). hESCs also produce Nodal, a TGFβ member that can signal through the Activin A/Alk4 receptor to activate Smad2,3. Wnt ligands produced by human pluripotent cells appear to be sufficient for activation of downstream targets such as β-catenin and Snail in the absence of PI3K/Akt signaling, although addition of exogenous Wnt3a has been reported to enhance rates and efficiencies of mesendoderm differentiation (D’Amour et al., 2005).
Together, our studies show that PI3K/Akt regulates the ability of Activin A/Smad2,3 to control the balance between self-renewal and differentiation (see Figure 7). Under self-renewing conditions, PI3K/Akt suppresses Erk and Wnt signaling, allowing Smad2,3 to activate a specific subset of target genes required for self-renewal. In the absence of PI3K/Akt signaling however, Erk and Wnt pathways are activated and effectors such as β-catenin and Snail can permit Smad2,3 to activate genes that direct early differentiation and EMT. Besides the activation of Wnt signaling, loss of PI3K increases the threshold of phosphorylated Smad2,3, enabling it to target a subset of genes that are not active under self-renewing conditions. Precisely how Akt regulates Smad2,3 thresholds is unclear, but may involve the direct interaction and sequestration of Smad3 out of the nucleus (Conery et al., 2004; Remy et al., 2004). How pluripotency genes become inactivated under these conditions is also unclear but could involve a negative feedback loop of some kind once early differentiation genes are activated. Interestingly, pluripotency factors such as Nanog and Oct4 have been recently implicated in the initiation of differentiation (Teo, et al., 2011; Yu et al., 2011; Thomson et al., 2011), which may account for their continued expression during the initial stages of differentiation.
Despite its well-established role in antagonizing pluripotency in mESCs, the role of Erk in human pluripotent cells has been more open to question (Armstrong et al., 2006; Li et al., 2007; Ding et al., 2010; Na et al., 2010). While we find that elevated Erk activity promotes mesendoderm differentiation, we cannot rule out the possibility that low levels of Erk signaling are important for hESC maintenance. In this scenario Erk signaling would be maintained below a threshold level to permit self-renewal but, once this level is surpassed, mesendoderm differentiation would initiate. Recent work has suggested that Erk signaling may promote the expression of Nanog during the initial stages of mesendoderm differentiation (Yu et al., 2011). Our work is consistent with this finding.
For some time the exact roles of Fgf2 and Wnt signaling in maintenance of human pluripotent cells have remained unclear. We believe a confounding problem in dissecting the roles of many signaling pathways has been the complexity and inconsistencies of media formulations such as MEF-CM. In our assays, Fgf2 has no consistent effect on Akt, Erk or Gsk3β at low concentrations (F10). However, at high concentrations (F100) it regulates Akt, Erk and Gsk3β in a manner comparable to that of Igf-1 and heregulin. Several reports have described the need for high levels of Fgf2 in feeder-free media formulations, consistent with our findings (Levenstein et al., 2006; Yao et al., 2006). In our studies we routinely used heregulin in place of Fgf2 because it functions at low concentrations and therefore provided cost benefits but, cells can be maintained long-term (>10 passages) in AIF100 media (data not shown). Signaling in AIF100 and HAI media was indistinguishable. The reason for requiring two sources of PI3K activators for long-term self-renewal is not understood. One possibility is that sustained PI3K signaling requires multiple activators of PI3K. This is suggested by studies indicating that different receptor tyrosine kinases activate PI3K with different kinetics (see Zhang et al., 2002). Sustaining PI3K signaling over time may therefore require two activators with different activation kinetics.
We have addressed the role of Wnt/Gsk3β signaling in hESCs in numerous ways using, (1) multiple chemical inhibitors (BIO, GSKi-XV, CHIR99021), (2) the Wnt antagonist, Dkk1 and (3) a genetic approach using a DN-GSK3. Additionally, these analyses were performed using multiple cell lines and multiple media conditions (HAI, StemPro®, MEF-CM, mTesr1®). Collectively we find that Wnt signaling is antagonistic to self-renewal and promotes differentiation of hESCs, but only if Erk and Activin/Smad signaling pathways are active.
Our experiments show that different concentrations of Gsk3 inhibitors have different biological effects and reconcile conflicting reports in the literature (Sato et al., 2004; James et al., 2005; Xiao et al., 2006; Villa Diaz et al., 2009; Ding et al., 2010; Dravid et al., 2005; Sumi et al., 2008; Hay et al., 2008; Bone et al., 2011). These findings are in agreement with a recent report showing that Gsk3 inhibitors have dose-dependent effects; at low concentrations they stabilize pluripotent cells and at high concentrations they promote differentiation (Tsutsui et al., 2011; Li et al., 2011). At the biochemical level, these findings can be explained by the presence of different Gsk3 complexes that perform separate biochemical functions (Voskas et al., 2011). In this scheme, the biological effects of separate Gsk3 complexes would be subject to different signaling thresholds. In pluripotent cells, Myc is stabilized at low concentrations of inhibitor, while β-catenin target gene activation requires higher concentrations. Here, Gsk3 complexes controlling Myc are part of the canonical PI3K/Akt pathway and serve to antagonize self-renewal pathways (Singh and Dalton, 2009; Cartwright et al., 2005; Smith et al., 2010; Takahashi and Yamanaka, 2006). Increased Myc stability therefore provides an explanation for how hESC self-renewal is promoted at low inhibitor concentrations. High concentrations of Gsk3 inhibitors activate β-catenin resulting in loss of pluripotency markers and increased levels of mesendoderm markers, consistent with the well-established role of Wnt in differentiation and development. The pool of Gsk3 required for this is part of the canonical Wnt pathway and quite separate from that which regulates substrates such as Myc. Together, these findings explain many of the discrepancies in the literature as to the function of Wnt/β-catenin signaling and Gsk3 inhibitors in hESCs.
Recent studies suggest that β-catenin is not required to maintain the pluripotency of mESCs (Lyashenko et al., 2001; Wray et al., 2011). However, low levels of β-catenin may promote pluripotency of ‘naïve’ ESCs by blocking Tcf3-based repression (Yi et al., 2011), and not through β-catenin activation of target genes. Additional work has also found that inhibition of Wnt promotes the conversion of ‘naïve’ mESCs to ‘primed’ EpiSCs (ten Berge et al., 2011). Importantly, hESCs are considered to be in a ‘primed’ pluripotent state, similar to mouse EpiSCs (Tesar et al., 2007). Our data showing that inhibition of Wnt signaling by Dkk1 promotes hESC pluripotency is therefore in agreement with these recent findings.
A prediction of our model is that cross-talk signaling between the Erk and Wnt pathways also controls mesoderm induction during early vertebrate development. While the Raf/Mek/Erk and Wnt pathways have long been known to control early mesoderm induction in vertebrate embryogenesis through transcriptional regulators such as Eomesodermin, Xbra and Mix1 (Heasman, 2006), the relationship between these pathways has not been clearly established. Although PI3K/Akt is unlikely to be involved in this scenario, our studies in human pluripotent cells in vitro have allowed us to uncover what we propose to be a mechanism that broadly applies to mesendoderm induction in vivo. As an extension of our model, we propose that Erk activation crosstalks with Gsk3β to activate canonical Wnt signaling during mesoderm and endoderm induction. Furthermore, studies in Xenopus have shown that the ability of Activin to induce mesoderm in animal cap assays is dependent upon Erk activity (LaBonne and Whitman, 1994). While the molecular mechanism for this is unclear, we predict that Erk, in conjunction with Wnt, promotes β-catenin activation such that it may cooperate with Smad2,3 complexes to activate mesodermal gene expression during embryonic development.
In summary, we have defined a framework to explain how cell-signaling pathways coordinate the balance between self-renewal and differentiation. Central to this framework is the activity of PI3K/Akt which allows Activin A/Smad2,3 signaling to promote self-renewal. In the absence of PI3K signaling Smad2,3 collaborates with Wnt pathway effectors to promote differentiation. This model has far-reaching implications for cell fate commitment not only in pluripotent cells in vitro but also for cell fate determination in early embryonic development.
EXPERIMENTAL PROCEDURES
Cell Culture
hESCs (WA01, WA07, WA09, from WiCell, Madison, WI, and BG01, BG02 from Novocell, Athens, GA) and hiPSCs (Fib2-iPS4, from Dr. George Daley) were routinely maintained in HAI (basis for StemPro® hESC SFM, Invitrogen) or AIF100 media, by a variation to the method previously described (Wang et al., 2007).
Immunoblotting, Immunostaining, and qRT-PCR
Immunostaining and immunoblotting was performed as previously described (Smith et al., 2010; Bechard and Dalton, 2009) qRT-PCR was performed using Taqman assays (Applied Biosystems) on a iCycler (Bio-Rad), according to manufacturer instructions. qRT-PCR assays performed in triplicate, normalized to Gapdh, and analyzed according to the ΔΔCT method. Data is representative of multiple experiments.
Immunoprecipitation and Chromatin Immunoprecipitation
Immunoprecipitation assays were performed by lysis in Mammalian Cell Lysis Buffer (Bechard and Dalton, 2009) and detected with an anti-rabbit light chain-specific HRP-conjugated secondary antibody (Millipore). Chromatin immunoprecipitation assays were performed as previously described (Smith el al., 2010).
Supplementary Material
Acknowledgments
This work was supported by grants to SD from the National Institute of Child Health and Human Development (HD049647) and the National Institute for General Medical Sciences (GM75334). We would like to thank George Daley for providing hiPSCs and Julie Nelson for assistance with FACS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armstrong L, Hughes O, Yung S, Hyslop L, Stewart R, Wappler I, Peters H, Walter T, Stojkovic P, Evans J, et al. The role of PI3K/AKT, MAPK/ERK and NFkappabeta signalling in the maintenance of human embryonic stem cell pluripotency and viability highlighted by transcriptional profiling and functional analysis. Hum Mol Genet. 2006;15:1894–1913. doi: 10.1093/hmg/ddl112. [DOI] [PubMed] [Google Scholar]
- Beattie GM, Lopez AD, Bucay N, Hinton A, Firpo MT, King CC, Hayek A. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells. 2005;23:489–495. doi: 10.1634/stemcells.2004-0279. [DOI] [PubMed] [Google Scholar]
- Bechard M, Dalton S. Subcellular localization of glycogen synthase kinase 3beta controls embryonic stem cell self-renewal. Mol Cell Biol. 2009;29:2092–2104. doi: 10.1128/MCB.01405-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock WL, Pearce M, Steelman LS, Franklin RA, McCarthy SA, Cherwinski H, McMahon M, McCubrey JA. A conditionally-active form of MEK1 results in autocrine tranformation of human and mouse hematopoietic cells. Oncogene. 2000;19:526–536. doi: 10.1038/sj.onc.1203337. [DOI] [PubMed] [Google Scholar]
- Bone HK, Nelson AS, Goldring CE, Tosh D, Welham MJ. A novel chemically directed route for the generation of definitive endoderm from human embryonic stem cells based on inhibition of GSK-3. J Cell Sci. 2011;124:1992–2000. doi: 10.1242/jcs.081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright P, McLean C, Sheppard A, Rivett D, Jones K, Dalton S. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development. 2005;132:885–896. doi: 10.1242/dev.01670. [DOI] [PubMed] [Google Scholar]
- Conery AR, Cao Y, Thompson EA, Townsend CM, Jr, Ko TC, Luo K. Akt interacts directly with Smad3 to regulate sensitivity to TGF-beta induced apoptosis. Nat Cell Biol. 2004;6:366–372. doi: 10.1038/ncb1117. [DOI] [PubMed] [Google Scholar]
- D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, Bartholomeusz G, Li Y, Pan Y, Li Z, Bargou RC, Qin J, Lai CC, Tsai FJ, Tsai CH, Hung MC. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell. 2005;19:159–70. doi: 10.1016/j.molcel.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Ding VM, Ling L, Natarajan S, Yap MG, Cool SM, Choo AB. FGF-2 modulates Wnt signaling in undifferentiated hESC and iPS cells through activated PI3-K/GSK3beta signaling. J Cell Physiol. 2010;225:417–428. doi: 10.1002/jcp.22214. [DOI] [PubMed] [Google Scholar]
- Dravid G, Ye Z, Hammond H, Chen G, Pyle A, Donovan P, Yu X, Cheng L. Defining the role of Wnt/beta-catenin signaling in the survival, proliferation, and self-renewal of human embryonic stem cells. Stem Cells. 2005;23:1489–1501. doi: 10.1634/stemcells.2005-0034. [DOI] [PubMed] [Google Scholar]
- Funaba M, Mathews LS. Identification and characterization of constitutively active Smad2 mutants: evaluation of formation of Smad complex and subcellular distribution. Mol Endocrinol. 2000;14:1583–1591. doi: 10.1210/mend.14.10.0537. [DOI] [PubMed] [Google Scholar]
- Hagen T, Di Daniel E, Culbert AA, Reith AD. Expression and characterization of GSK-3 mutants and their effect on beta-catenin phosphorylation in intact cells. J Biol Chem. 2002;277:23330–23335. doi: 10.1074/jbc.M201364200. [DOI] [PubMed] [Google Scholar]
- Harvey CD, Ehrhardt AG, Cellurale C, Zhong H, Yasuda R, Davis RJ, Svoboda K. A genetically encoded fluorescent sensor of ERK activity. Proc Natl Acad Sci U S A. 2008;105:19264–19269. doi: 10.1073/pnas.0804598105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay DC, Fletcher J, Payne C, Terrace JD, Gallagher RC, Snoeys J, Black JR, Wojtacha D, Samuel K, Hannoun Z, et al. Highly efficient differentiation of hESCs to functional hepatic endoderm requires ActivinA and Wnt3a signaling. Proc Natl Acad Sci U S A. 2008;105:12301–12306. doi: 10.1073/pnas.0806522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman J. Patterning the early Xenopus embryo. Development. 2006;133:1205–1217. doi: 10.1242/dev.02304. [DOI] [PubMed] [Google Scholar]
- James D, Levine AJ, Besser D, Hemmati-Brivanlou A. TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132:1273–1282. doi: 10.1242/dev.01706. [DOI] [PubMed] [Google Scholar]
- LaBonne C, Whitman M. Mesoderm induction by activin requires FGF-mediated intracellular signals. Development. 1994;120:463–472. doi: 10.1242/dev.120.2.463. [DOI] [PubMed] [Google Scholar]
- Levenstein ME, Ludwig TE, Xu RH, Llanas RA, VanDenHeuvel-Kramer K, Manning D, Thomson JA. Basic fibroblast growth factor support of human embryonic stem cell self-renewal. Stem Cells. 2006;24:568–574. doi: 10.1634/stemcells.2005-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang G, Wang C, Zhao Y, Zhang H, Tan Z, Song Z, Ding M, Deng H. MEK/ERK signaling contributes to the maintenance of human embryonic stem cell self-renewal. Differentiation. 2007;75:299–307. doi: 10.1111/j.1432-0436.2006.00143.x. [DOI] [PubMed] [Google Scholar]
- Li W, Jiang K, Ding S. A Chemical Approach to Controlling Cell Fate and Function. Stem Cells. 2011 doi: 10.1002/stem.768. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, Frane JL, Crandall LJ, Daigh CA, Conard KR, Piekarczyk MS, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- Lyashenko N, Winter M, Migliorini D, Biechele T, Moon RT, Hartmann C. Differential requirement for the dual functions of β-catenin in embryonic stem cell self-renewal and germ layer formation. Nat Cell Biol. 2011;13:753–761. doi: 10.1038/ncb2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Hoyle PE, Blalock WL, Weinstein-Oppenheimer C, Franklin RA, Cherwinski H, Bosch E, McMahon M. Differential abilities of activated Raf oncoproteins to abrogate cytokine dependency, prevent apoptosis and induce autocrine growth factor synthesis in human hematopoietic cells. Leukemia. 1998;12:1903–1929. doi: 10.1038/sj.leu.2401215. [DOI] [PubMed] [Google Scholar]
- McLean AB, D’Amour KA, Jones KL, Krishnamoorthy M, Kulik MJ, Reynolds DM, Sheppard AM, Liu H, Xu Y, Baetge EE, Dalton S. Activin a efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells. 2007;25:29–38. doi: 10.1634/stemcells.2006-0219. [DOI] [PubMed] [Google Scholar]
- Menendez L, Yatskievych TA, Antin PB, Dalton S. Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proc Natl Acad Sci USA. 2011;108:19240–19245. doi: 10.1073/pnas.1113746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na J, Furue MK, Andrews PW. Inhibition of ERK1/2 prevents neural and mesendodermal differentiation and promotes human embryonic stem cell self-renewal. Stem Cell Res. 2010;5:157–169. doi: 10.1016/j.scr.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Ohtsuka S, Dalton S. Molecular and biological properties of pluripotent embryonic stem cells. Gene Ther. 2008;15:74–81. doi: 10.1038/sj.gt.3303065. [DOI] [PubMed] [Google Scholar]
- Remy I, Montmarquette A, Michnick SW. PKB/Akt modulates TGF-beta signaling through a direct interaction with Smad3. Nat Cell Biol. 2004;6:358–365. doi: 10.1038/ncb1113. [DOI] [PubMed] [Google Scholar]
- Rommel C, Clarke BA, Zimmermann S, Nuñez L, Rossman R, Reid K, Moelling K, Yancopoulos GD, Glass DJ. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science. 1999;286:1738–1741. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- Singh AM, Dalton S. The cell cycle and Myc intersect with mechanisms that regulate pluripotency and reprogramming. Cell Stem Cell. 2009;5:141–149. doi: 10.1016/j.stem.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KN, Singh AM, Dalton S. Myc represses primitive endoderm differentiation in pluripotent stem cells. Cell Stem Cell. 2010;7:343–354. doi: 10.1016/j.stem.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm MP, Bone HK, Beck CG, Bourillot PY, Schreiber V, Damiano T, Nelson A, Savatier P, Welham MJ. Regulation of Nanog expression by phosphoinositide 3-kinase-dependent signaling in murine embryonic stem cells. J Biol Chem. 2007;282:6265–6273. doi: 10.1074/jbc.M610906200. [DOI] [PubMed] [Google Scholar]
- Sumi T, Tsuneyoshi N, Nakatsuji N, Suemori H. Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical Wnt/beta-catenin, Activin/Nodal and BMP signaling. Development. 2008;135:2969–2979. doi: 10.1242/dev.021121. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- ten Berge D, Kurek D, Blauwkamp T, Koole W, Maas A, Eroglu E, Siu RK, Nusse R. Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat Cell Biol. 2011;13:1070–1075. doi: 10.1038/ncb2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo AK, Arnold SJ, Trotter MW, Brown S, Ang LT, Chng Z, Robertson EJ, Dunn NR, Vallier L. Pluripotency factors regulate definitive endoderm specification through eomesodermin. Genes Dev. 2011;25:238–250. doi: 10.1101/gad.607311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- Thomson M, Liu SJ, Zou LN, Smith Z, Meissner A, Ramanathan S. Pluripotency factors in embryonic stem cells regulate differentiation into germ layers. Cell. 2011;145:875–889. doi: 10.1016/j.cell.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui H, Valamehr B, Hindoyan A, Qiao R, Ding X, Guo S, Witte ON, Liu X, Ho CM, Wu H. An optimized small molecule inhibitor cocktail supports long-term maintenance of human embryonic stem cells. Nat Commun. 2011;25:2–167. doi: 10.1038/ncomms1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- Vallier L, Mendjan S, Brown S, Chng Z, Teo A, Smithers LE, Trotter MW, Cho CH, Martinez A, Rugg-Gunn P, et al. Activin/Nodal signalling maintains pluripotency by controlling Nanog expression. Development. 2009;136:1339–1349. doi: 10.1242/dev.033951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Diaz LG, Pacut C, Slawny NA, Ding J, O’Shea KS, Smith GD. Analysis of the factors that limit the ability of feeder cells to maintain the undifferentiated state of human embryonic stem cells. Stem Cells Dev. 2009;18:641–651. doi: 10.1089/scd.2008.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskas D, Ling LS, Woodgett JR. Does GSK-3 provide a shortcut for PI3K activation of Wnt signalling? F1000. Biol Rep. 2010;2:82. doi: 10.3410/B2-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Schulz TC, Sherrer ES, Dauphin DS, Shin S, Nelson AM, Ware CB, Zhan M, Song CZ, Chen X, et al. Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signaling. Blood. 2007;110:4111–4119. doi: 10.1182/blood-2007-03-082586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle FC, Smith JC. Transcriptional regulation of mesendoderm formation in Xenopus. Semin Cell Dev Biol. 2006;17:99–109. doi: 10.1016/j.semcdb.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Umehara H, Murayama K, Okabe M, Kimura T, Nakano T. Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene. 2006;25:2697–2707. doi: 10.1038/sj.onc.1209307. [DOI] [PubMed] [Google Scholar]
- Weisberg E, Winnier GE, Chen X, Farnsworth CL, Hogan BL, Whitman M. A mouse homologue of FAST-1 transduces TGF beta superfamily signals and is expressed during early embryogenesis. Mech Dev. 1998;79:17–27. doi: 10.1016/s0925-4773(98)00160-9. [DOI] [PubMed] [Google Scholar]
- Wray J, Kalkan T, Smith AG. The ground state of pluripotency. Biochem Soc Trans. 2010;38:1027–1032. doi: 10.1042/BST0381027. [DOI] [PubMed] [Google Scholar]
- Wray J, Kalkan T, Gomez-Lopez S, Eckardt D, Cook A, Kemler R, Smith A. Inhibition of glycogen synthase kinase-3 alleviates Tcf3 repression of the pluripotency network and increases embryonic stem cell resistance to differentiation. Nat Cell Biol. 2011;13:838–845. doi: 10.1038/ncb2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Yuan X, Sharkis SJ. Activin A maintains self-renewal and regulates fibroblast growth factor, Wnt, and bone morphogenic protein pathways in human embryonic stem cells. Stem Cells. 2006;24:1476–1486. doi: 10.1634/stemcells.2005-0299. [DOI] [PubMed] [Google Scholar]
- Xu RH, Sampsell-Barron TL, Gu F, Root S, Peck RM, Pan G, Yu J, Antosiewicz-Bourget J, Tian S, Stewart R, Thomson JA. NANOG is a direct target of TGFbeta/activin-mediated SMAD signaling in human ESCs. Cell Stem Cell. 2008;3:196–206. doi: 10.1016/j.stem.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Chen S, Clark J, Hao E, Beattie GM, Hayek A, Ding S. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci U S A. 2006;103:6907–6912. doi: 10.1073/pnas.0602280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Pereira L, Hoffman JA, Shy BR, Yuen CM, Liu DR, Merrill BJ. Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal. Nat Cell Biol. 2011;13:762–770. doi: 10.1038/ncb2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Pan G, Yu J, Thomson JA. FGF2 Sustains NANOG and Switches the Outcome of BMP4-Induced Human Embryonic Stem Cell Differentiation. Cell Stem Cell. 2011;8:326–34. doi: 10.1016/j.stem.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SQ, Tsiaris WT, Araki T, Wen G, Minichiello L, Klein R, Neel BJ. Receptor-specific regulation of phosphatidylinositol 3′-kinase activation by the protein tyrosine phosphatase Shp2. Mol Cell Biol. 2002;22:4062–4072. doi: 10.1128/MCB.22.12.4062-4072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.