Abstract
During cell division, the activation of glycolysis is tightly regulated by the action of two ubiquitin ligases, anaphase-promoting complex/cyclosome–Cdh1 (APC/C-Cdh1) and SKP1/CUL-1/F-box protein–β-transducin repeat-containing protein (SCF-β-TrCP), which control the transient appearance and metabolic activity of the glycolysis-promoting enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase, isoform 3 (PFKFB3). We now demonstrate that the breakdown of PFKFB3 during S phase occurs specifically via a distinct residue (S273) within the conserved recognition site for SCF-β-TrCP. Glutaminase 1 (GLS1), the first enzyme in glutaminolysis, is also targeted for destruction by APC/C-Cdh1 and, like PFKFB3, accumulates after the activity of this ubiquitin ligase decreases in mid-to-late G1. However, our results show that GLS1 differs from PFKFB3 in that its recognition by APC/C-Cdh1 requires the presence of both a Lys-Glu-Asn box (KEN box) and a destruction box (D box) rather than a KEN box alone. Furthermore, GLS1 is not a substrate for SCF-β-TrCP and is not degraded until cells progress from S to G2/M. The presence of PFKFB3 and GLS1 coincides with increases in generation of lactate and in utilization of glutamine, respectively. The contrasting posttranslational regulation of PFKFB3 and GLS1, which we have verified by studies of ubiquitination and protein stability, suggests the different roles of glucose and glutamine at distinct stages in the cell cycle. Indeed, experiments in which synchronized cells were deprived of either of these substrates show that both glucose and glutamine are required for progression through the restriction point in mid-to-late G1, whereas glutamine is the only substrate essential for the progression through S phase into cell division.
Cell division is regulated by the anaphase-promoting complex/cyclosome (APC/C), a large multimeric ubiquitin ligase that targets key mitotic regulators for destruction by the proteasome. APC/C identifies substrates for ubiquitination by using the activator proteins Cdc20 or Cdh1 to recognize specific degradation motifs within target proteins (1). APC/C-Cdc20 regulates proteins involved in metaphase-to-anaphase transition, whereas APC/C-Cdh1 is responsible for the maintenance of G1 through the degradation of a number of proteins, including S-phase cyclins (2, 3). Inactivation of APC/C-Cdh1 in mid-to-late G1 is necessary for G1-to-S transition. We have recently established that APC/C-Cdh1 also degrades two key enzymes in the metabolic pathways of glycolysis and glutaminolysis, namely 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase, isoform 3 (PFKFB3) (4) and glutaminase 1 (GLS1) (5), respectively. These findings explain the molecular connection between cell-cycle progression and the provision of nutrients essential for this purpose; they also account for the nutrient-dependent restriction point in late G1 (6, 7). We have obtained similar results with human T lymphocytes (5), embryonically-derived kidney cells (HEK293), and neoplastic neuroblastoma cells (4), indicating that the phenomenon is common to normal and transformed proliferating cells.
Several APC/C degradation motifs have been characterized, including the destruction box (D box) and the Lys-Glu-Asn box (KEN box). The D box, with the consensus amino acid sequence of [RH]xxLxx[LIVM] (where x indicates any amino acid), is found in many APC/C substrates, including mitotic cyclins, and is essential for their ubiquitin-mediated destruction (8). The KEN box is also found in several APC/C substrates and is preferentially, but not exclusively, recognized by APC/C-Cdh1 (9). PFKFB3 is degraded by APC/C-Cdh1 through its recognition of a KEN box present in this enzyme (10), and initial studies with GLS1 showed that its degradation by this ubiquitin ligase was through the recognition of a C-terminal region containing a KEN box (5). However, bioinformatic analysis shows that the C-terminal region also contains a D box (11), and it has become clear that, in certain proteins, both a KEN box and a D box are necessary for recognition by APC/C-Cdh1 (12). We have therefore generated a series of constructs of GLS1 in which we have mutated the KEN box, the D box, and both of these destruction motifs in the C-terminal region of the enzyme to elucidate the specific recognition site in GLS1 for targeting by APC/C-Cdh1.
Our previous studies in synchronized HeLa cells demonstrated that the appearance of PFKFB3 in mid-to-late G1 is essential for cell division because its silencing prevents progression into S phase. We also found that PFKFB3 ceases to be detectable during late G1/S despite the absence of Cdh1 and showed that this disappearance was attributable to the action of SKP1/CUL-1/F-box protein–β-transducin repeat-containing protein (SCF-β-TrCP) (7). This ubiquitin ligase is active during S phase (13) and recognizes a conserved DSGXXS degradation site (DSG box) present in PFKFB3 (7). There is a requirement for the substrates of SCF-β-TrCP to be phosphorylated (14, 15). In PFKFB3, a distinct phosphorylation site, serine273, has been identified (16) that is different from those phosphorylated by AMP-activated protein kinase (AMPK) or Akt. S273 is located within the PFKFB3 DSG box, and we have now investigated whether its phosphorylation is required for recognition of the DSG box by SCF-β-TrCP. Our results clarify the mechanisms that control the kinetics of appearance and disappearance, and thus the “cycling,” of PFKFB3 and GLS1 during cell division as well as their respective metabolic activities in terms of lactic acid generation and glutamine utilization. In addition, by selectively removing either glucose or glutamine from the medium of HeLa cells synchronized by double thymidine block (DTB) alone or by DTB plus nocodazole, we have established the requirement for these substrates at two distinct stages of the cell cycle.
Results
Appearance of PFKFB3 and GLS1 During the Cell Cycle and Its Metabolic Consequences.
HeLa cells synchronized with DTB and nocodazole were released from mitotic arrest. Immunoblotting of cell extracts at different times after release established that the amount of PFKFB3 protein was initially barely detectable, then increased for a brief period (at 6–8 h) before decreasing to background levels (Fig. 1A, Left). The amount of GLS1 protein also increased after 6 h but remained high throughout the observation period (12 h). The appearance of both PFKFB3 and GLS1 coincided with the reduction at 6 h of the APC/C activator protein Cdh1, which subsequently ceased to be detectable (Fig. 1A, Left). Cell-cycle phase analysis by FACS (Fig. 1B, Left) established that the amount of PFKFB3 peaked in mid-to-late G1, after which it disappeared, remaining below the detection limit for the rest of the cell cycle, as demonstrated in cells released from DTB at G1/S (Fig. 1A, Right). In contrast, GLS1 was present in cells released from DTB and persisted throughout S phase, gradually declining as cells progressed into G2/M and coincident with the reappearance of Cdh1 (Fig. 1 A and B, Right). After release from nocodazole, the rate of lactate production correlated with the appearance of PFKFB3, peaking at 6–8 h (Fig. 1C, Left) and returning to basal levels for the rest of the cycle, as seen in cells released from DTB (Fig. 1C, Right). In contrast, glutamine uptake from the medium increased at 6 h after release from nocodazole (Fig. 1D, Left) and remained elevated for the rest of the cycle, as seen in cells released from DTB. Glutamine uptake returned to basal levels as Cdh1 reappeared (Fig. 1D, Right), closely following the pattern of GLS1 expression (Fig. 1A).
Fig. 1.
Changes in protein levels of PFKFB3 and GLS1 during the cell cycle and their metabolic consequences. (A) HeLa cells were released from DTB plus nocodazole (Left) or DTB alone (Right). Whole-cell extracts from synchronized cells were subjected to immunoblotting at the indicated times after release. (B) The cell-cycle profile of the cells at different times after release, as determined by FACS analysis of DNA content. (C) The lactate production rate at different times during the cell cycle. The rate was determined as the difference at each time from the previous measurement. (D) The rate of utilization of glutamine at different times during the cell cycle. The rate was determined as the difference at each time from the previous measurement. (A) Representative images and mean densitometry values from three independent experiments. (B) Mean of three independent experiments. (C and D) Mean ± SEM, n = 3. *P < 0.05.
Effect of β-TrCP on the Stability of PFKFB3 and GLS1.
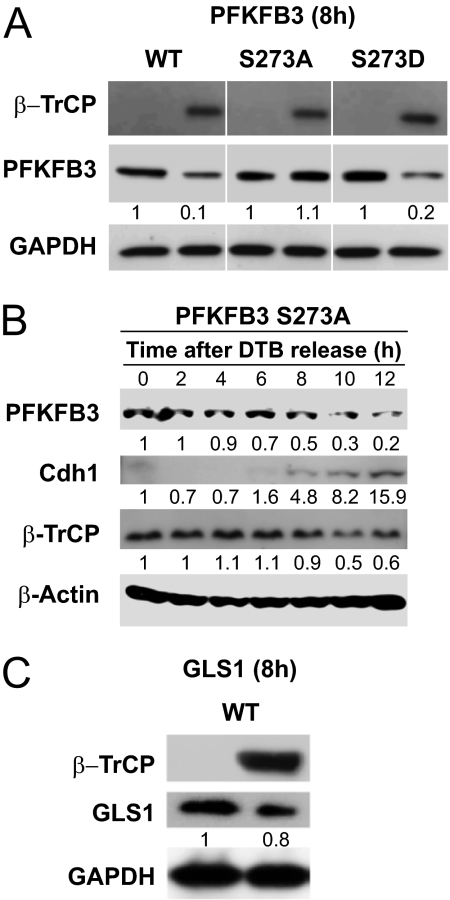
To determine whether S273 is the specific residue whose phosphorylation is required for recognition of PFKFB3 by SCF-β-TrCP, we have used a protein form in which this serine residue was mutated to alanine (S273A), thus preventing phosphorylation of the site, and one in which it was mutated to aspartate (S273D), thus imitating phosphorylation. We found that when wild-type PFKFB3 was coexpressed with β-TrCP in HeLa cells synchronized with DTB and nocodazole, the enzyme was degraded at 8 h after release (Fig. 2A). The stability of PFKFB3 S273A was, however, unaffected by coexpression of β-TrCP, whereas S273D was degraded in similar experimental conditions (Fig. 2A). These results show the requirement for phosphorylation of S273 in the degradation of PFKFB3 by SCF-β-TrCP (Fig. 2A). Indeed, when PFKFB3 S273A was overexpressed in cells synchronized with DTB, the protein was initially stable after release, despite the presence of β-TrCP, but began to be degraded at later stages, when Cdh1 appeared (Fig. 2B). GLS1, which lacks a consensus DSG motif, was not affected by overexpression of β-TrCP under similar experimental conditions (Fig. 2C).
Fig. 2.
Effect of β-TrCP on the stability of PFKFB3 and GLS1. (A) The susceptibility of different constructs of PFKFB3 to degradation by β-TrCP was determined in HeLa cells synchronized with DTB and nocodazole. Wild-type PFKFB3, a protein form in which S273 was mutated to alanine (S273A), and one in which it was mutated to aspartate (S273D) were expressed in the presence or absence of coexpressed β-TrCP. Cell lysates were subjected to immunoblotting at 8 h after release from nocodazole. (B) Stability of PFKFB3 S273A after release from DTB. PFKFB3 S273A was overexpressed in HeLa cells synchronized with DTB alone, and the amount of PFKFB3, Cdh1, and β-TrCP proteins was measured by immunoblotting at different times after release. (C) GLS1 was expressed in cells synchronized with DTB and nocodazole in the presence or absence of coexpressed β-TrCP, and the protein content was determined at 8 h after release. Representative images and mean densitometry values from three independent experiments.
Identification of the Recognition Site for Cdh1 in GLS1.
We have previously shown that overexpressed Cdh1 breaks down coexpressed wild-type GLS1 (KENwt D boxwt) but does not affect the enzyme if the last 14 aa are deleted (ΔGLS1) (ref. 5 and Fig. 3A). This C-terminal region contains both a KEN box and a modified D box (i.e., HxxLxxL; Fig. 3B). To establish the specific recognition site for APC/C-Cdh1 in GLS1, we transfected asynchronous HEK293 cells with wild-type GLS1 or with enzyme in which the KEN box, the D box, or both destruction motifs had been modified by site-directed mutagenesis (Fig. 3B). Coexpression of Cdh1 in these transfected cells resulted in almost complete disappearance of all constructs apart from that in which both the KEN and D boxes were mutated (GLS1 KENmut D boxmut; Fig. 3C). We confirmed that these motifs were involved in the degradation of GLS1 by determining the stability of the wild-type and mutated GLS1 proteins in transfected asynchronous HEK293 cells in which protein synthesis was blocked by cycloheximide. The amount of protein decreased with time in cells transfected with wild-type GLS1 but not in those transfected with ΔGLS1 or GLS1 KENmut D boxmut (Fig. 3 D and E).
Fig. 3.
Identification of the recognition site for Cdh1 in GLS1. (A) The susceptibility of GLS1 KENwt D boxwt and ΔGLS1 (in which the last 14 aa are deleted) to degradation by coexpressed Cdh1 was determined in asynchronous HEK293 cells. (B) Illustration of wild-type GLS1 and various mutations carried out in the KEN box, the D box, and in both recognition sites. (C) The susceptibility of the different mutations of GLS1 to degradation by coexpressed Cdh1. (D) The stability of wild-type GLS1 and ΔGLS1 in asynchronous HEK293 cells in which protein synthesis was blocked by cycloheximide. (E) The stability of wild-type GLS1 and GLS1 KENmut D boxmut in asynchronous HEK293 cells in which protein synthesis was blocked by cycloheximide. (A and C–E) Representative images and mean densitometry values from three independent experiments.
Metabolism of PFKFB3 and GLS1 by Ubiquitination.
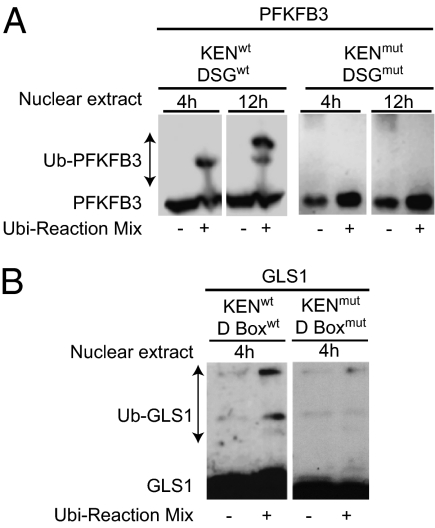
Ubiquitination of PFKFB3, PFKFB3 KENmut DSGmut, GLS1, and GLS1 KENmut D boxmut was investigated in vitro. HeLa cells overexpressing these enzymes were synchronized with DTB and nocodazole, and the proteins were extracted at 8 h after release. Enriched nuclear fractions of synchronized HeLa cells obtained at 4 h and 12 h after release from nocodazole were used as sources of activity of APC/C-Cdh1 and SCF-β-TrCP, respectively. These fractions were added to the extracted proteins, in the presence or absence of ubiquitination reaction mix, and the shift in mobility of the protein after ubiquitination was monitored by immunoblotting. Wild-type PFKFB3 was ubiquitinated by nuclear extract obtained at both 4 h and 12 h after release from nocodazole, indicating that it is a substrate for both APC/C-Cdh1 and SCF-β-TrCP, whereas the double mutant (PFKFB3 KENmut DSGmut) was not modified by either extract (Fig. 4A). GLS1 was ubiquitinated by the 4-h (APC/C-Cdh1–containing) extract, and this effect was prevented by mutation in both the KEN and the D boxes (Fig. 4B).
Fig. 4.
Ubiquitination of PFKFB3 and GLS1. (A) The ubiquitination of PFKFB3 wild-type and double mutant (KENmut DSGmut) was investigated in vitro. Enriched nuclear fractions of synchronized HeLa cells obtained at 4 h and 12 h after release from nocodazole were used as sources of activity of APC/C-Cdh1 and SCF-β-TrCP, respectively. These fractions were added to the extracted proteins, in the presence or absence of ubiquitination reaction mix, and the shift in mobility of the protein after ubiquitination was monitored by immunoblotting. (B) The ubiquitination of GLS1 wild-type and double mutant (KENmut D boxmut) was investigated in vitro as described in A. Images are representative of three independent experiments.
Requirement for Glucose and Glutamine During the Cell Cycle.
We also investigated whether glucose and glutamine are necessary for progression from G1 to S (with HeLa cells synchronized by DTB plus nocodazole) or for completion of the cell cycle beyond the restriction point (with cells synchronized by DTB alone). In cells synchronized by DTB plus nocodazole and then released into a medium containing no glucose, progression from G1 to S did occur but somewhat later than it did in control cells (Fig. 5 A and B, Left). In contrast, cells released from nocodazole into medium containing no glutamine remained predominantly in G1 and did not progress to S phase even after 18 h (Fig. 5C, Left). When released from DTB alone into medium lacking glucose, the cells completed their cycle and appeared to be the same as control cells throughout the observation period (up to 12 h; Fig. 5 A and B, Right). However, when such cells were released into medium lacking glutamine, they did not progress from S to G2/M even after 12 h (Fig. 5C, Right). The cell viability at the end of the observation period was >92% in all treatment groups.
Fig. 5.
Requirement for glucose and glutamine during the cell cycle. (A) Cell-cycle progression in HeLa cells synchronized by DTB plus nocodazole (Left) or by DTB alone (Right) then released into medium containing 5 mM glucose and 4 mM glutamine. (B) Cell-cycle progression in cells synchronized by DTB plus nocodazole (Left) or by DTB alone (Right) then released into medium containing no glucose. (C) Cell-cycle progression in cells synchronized by DTB plus nocodazole (Left) or by DTB alone (Right) then released into medium containing no glutamine. All results shown are representative of three to four independent experiments.
Further experiments were carried out in HeLa cells, synchronized by DTB plus nocodazole or by DTB alone, in which PFKFB3 or GLS1 was silenced (Fig. S1). Silencing PFKFB3 (siRNA), as verified by a reduction in the mRNA and protein levels, prevented cells synchronized by DTB plus nocodazole from progressing from G1 to S (Fig. S1A) but did not prevent such progression in cells synchronized by DTB alone (Fig. S1B). Silencing GLS1 (shRNA), as verified by a reduction in the mRNA and protein levels, prevented transition from G1 to S in cells synchronized by DTB plus nocodazole (Fig. S1C) and held them in S phase in cells synchronized by DTB alone (Fig. S1D).
To investigate why progression from G1 to S phase could occur in synchronized cells (DTB plus nocodazole) lacking glucose, albeit more slowly than in cells in normal medium, but not in those in which PFKFB3 had been silenced, cells lacking glucose were treated with the glycogen phosphorylase inhibitor CP-91149 (20 μM) (17) to prevent glycogenolysis. The inhibitor prevented transition from G1 to S in cells lacking glucose and synchronized with DTB plus nocodazole (Fig. S2A) but had no effect on cell-cycle progression in such cells synchronized with DTB alone (Fig. S2B).
Discussion
We have recently found that PFKFB3 and GLS1, critical enzymes for glycolysis and glutaminolysis during cell proliferation, are regulated by decreases in APC/C-Cdh1 (4, 5, 7). This finding has led us to suggest that the activity of these enzymes and its consequences represent the nutrient-sensitive restriction point in cell proliferation. In the case of PFKFB3, we found a further step of control provided by the ubiquitin ligase SCF-β-TrCP, which, by acting on a DSG consensus site present in PFKFB3, rapidly degrades it after its peak activity at ∼8 h after release from synchronization by DTB and nocodazole. The brief presence of PFKFB3 coincides with a peak in glycolytic activity, as demonstrated by a sharp and relatively short-lasting release of lactic acid (this paper and ref. 7).
The ubiquitin ligase SCF-β-TrCP is known to recognize substrates with phosphorylated DSG motifs (14, 15). We have previously speculated that S273 might be the phosphorylation site in PFKFB3 (7) because it is located within the DSG box of the enzyme and has been shown to be phosphorylated in proliferating T cells (16). We have now studied the susceptibility to SCF-β-TrCP of PFKFB3 constructs in which S273 has been mutated either to alanine (S273A), to prevent phosphorylation of the site, or to aspartate (S273D), to imitate phosphorylation (18). These studies show that PFKFB3 S273A is not a substrate for SCF-β-TrCP under conditions in which wild-type and PFKFB3 S273D are degraded, thus indicating the relevance of S273 for recognition of PFKFB3 by this ubiquitin ligase. Several kinases are known to phosphorylate DSG degradation sites in other proteins, including glycogen synthase kinase 3 (GSK3), polo-like kinase 1 (Plk1), and ribosomal protein S6 kinase β-1 (S6K1) (19, 20). Whether any of these kinases play a role in the degradation of PFKFB3 through β-TrCP requires further investigation.
We have extended our earlier studies of GLS1 (5) and now show that not only the KEN box but also the adjacent D box is required for recognition by APC/C-Cdh1. Such requirement for both destruction boxes has previously been demonstrated in other targets of this ubiquitin ligase, including CDC6 (21), which is a key regulator of the initiation of DNA replication, and Sgo1 (12), which plays an important role in the protection of centromeric adhesion. Furthermore, we show that, unlike PFKFB3, GLS1 is not targeted for destruction by SCF-β-TrCP. GLS1 remains detectable throughout S phase and does not decline until after the reappearance of Cdh1 during the later stages of the cell cycle (G2/M). These differences in duration of PFKFB3 and GLS1 are reflected in their activity, so that the rate of lactate production (an indicator of glycolysis and therefore, indirectly, of PFKFB3 activity) peaks during mid-to-late G1 and then declines, whereas the rate of glutamine utilization (an indicator of glutaminolysis and thus of GLS1 activity) increases during mid-to-late G1 and remains elevated until the end of G2/M.
These and our previous results (5, 7) therefore demonstrate that certain metabolic proteins, of which PFKFB3 and GLS1 might just be prominent examples, are regulated during the cell cycle in the same way as the cyclins. Their appearance and disappearance at different stages determine the metabolic behavior of the cell during division and, as a consequence, during cell proliferation. Understanding the kinetics of the cycling of these enzymes and their consequences offers a rational basis for the study of cell metabolism in proliferating cells, both normal and cancerous.
In relation to metabolism, the present results suggest that there are two critical points during the cell cycle: first, the passage through the restriction point in mid-to-late G1 and, second, the biosynthetic S phase. We show that, after release from nocodazole in G2/M, which allows investigation of the restriction point, both glutamine and glucose are necessary since the absence of either substrate or the silencing of PFKFB3 or GLS1 interferes with progression into S phase. Cells deprived of glucose are able to progress from G1 to S phase after a delay; however, this transition can be prevented by CP-91149, suggesting that such cells may be able to obtain sufficient glucose from glycogen to accomplish this step. Indeed, HeLa cells, which depend highly on glutamine for proliferation (22), are also known to accumulate significant amounts of glycogen when grown in the presence of glucose (23). A different scenario is observed when movement through S phase into G2/M is studied in cells released from DTB into G1 beyond the restriction point. These cells, in the absence of glucose, go through S phase and enter G2/M at the same rate as those in which both substrates are present. The same effect is observed if PFKFB3 is silenced during this stage. However, in the absence of glutamine, the cells enter S phase but fail to progress into G2/M, an effect also observed in cells in which GLS1 is silenced. Thus, although both glucose and glutamine are required for transition from G1 to S phase, once this transition has been accomplished, the cells are able to progress into cell division using only glutamine, as shown in the experiments in which they are released from DTB in the presence or absence of CP-91149. Indeed, glutamine, through its utilization in the Krebs cycle, seems to be able to generate all of the precursors, reducing equivalents and energy required for cell division (24).
The specific relevance of glucose at the restriction point requires further investigation. It is known that only a small percentage (<10%) of glucose (and indeed of glutamine) that is taken up is used in biosynthetic reactions in cancer cells (22, 25) or in normal proliferating cells (26, 27). This finding led, many years ago, to the suggestion that the accelerated utilization of glucose and glutamine during proliferation was necessary to keep downstream synthetic steps adequately provided with precursors (27). This is the most satisfactory explanation for accelerated glycolysis and glutaminolysis that has been proposed to date. The glucose that is not converted to lactate during G1 to S phase could be used in other reactions, including nucleotide biosynthesis via the nonoxidative branch of the pentose phosphate pathway [in cooperation with a reduction in the activity of pyruvate kinase type M2 (28)], fatty acid synthesis, which has been shown to be mainly supported through citrate production from glucose carbons (25), amino acid synthesis, and glycerogenesis. In addition to these well-known pathways, it has recently been suggested that glucose may play a role in cell signaling and gene expression. Thus, glucose, together with glutamine, is necessary for the formation of hexosamine, which is important in modifying proteins and lipids for their role in signal transduction (29). Furthermore, histone acetylation, and therefore gene expression, has been linked to glucose utilization through the actions of ATP-citrate lyase (30).
It is clear from our results that quantitative metabolic studies on the conversion of these two key substrates at defined phases of the cell cycle are now required. Such investigations will clarify, in a way in which studies on nonsynchronized proliferating cells have not been able to do, the precise role of each precursor and the way in which their utilization is connected to the myriad metabolic pathways that are switched on during cell division. Direct comparison of how neoplastic and nonneoplastic cells use these mechanisms will also reveal whether our observations are characteristic of HeLa and other highly proliferative neoplastic cells or of proliferating cells in general. Such data will define opportunities for the discovery of drugs that tackle cancer metabolism without potential side effects related to their mechanism of action.
Materials and Methods
Cell Culture.
HeLa S3 cells (Invitrogen) were cultivated in DMEM supplemented with 10% heat-inactivated FBS and penicillin (100 U/mL)/streptomycin (100 μg/mL) at 37 °C in a 5% CO2 humidified incubator. For preparation of synchronous cell populations, cells were synchronized in G1/S by two sequential 24-h blocks in 3 mM thymidine (Sigma) separated by a 12-h interval without thymidine (DTB) (31). To synchronize cells in mitosis they were subsequently treated with 100 ng/mL nocodazole (Sigma) for a further 12 h (Noc). DMEM lacking both glucose and glutamine was obtained from Life Technologies. Cycloheximide and CP-91149 were obtained from Sigma.
Cell Transfection.
Plasmids.
pcDNA3.3 Gls1 and ΔGls1 as well as pcDNA3.3 Cdh1 were prepared as described by Colombo et al. (5). Site-directed mutagenesis of the KEN box in Gls1 was carried out according to the manufacturer's instructions (QuikChange II; Stratagene) with the primer 5′-GGAGATTCTGACAACGGGGCGGCAGCTCAAACAGTCCATAAGAAT-3′ (and its reverse and complementary primer; Invitrogen). Underlined nucleotides indicate the mutated amino acids (KEN→AAA). pcDNA3.3 Gls1 D box and Gls1 KEN/D box were generated synthetically, introducing the following amino acid changes: KEN to AAA and HKNLDGLL to AKNADGAA (Geneart; Life Technologies). pcDNA3.3 PFKFB3 and PFKFB3 DSG/KEN were prepared as described previously (7). pcDNA3.3 PFKFB3 S273A and S273D were generated synthetically (Geneart; Life Technologies). All plasmid DNAs used for transfection were prepared with a Plasmid Maxi Kit (Qiagen) following the manufacturer's protocol and then confirmed by sequencing. For details of cell transfection and determination of lactate and glutamine, see SI Materials and Methods.
Immunoblotting.
Aliquots (20–60 μg of protein) of purified HeLa cell lysates, obtained with RIPA buffer plus protease inhibitor mixture (Roche), were cleared by centrifugation (14,000 × g for 10 min). Supernatants were heated for 5 min at 95 °C under reducing conditions and then electrophoresed in a 4–20% SDS acrylamide gel (Life Technologies). Proteins were transferred electrophoretically to nitrocellulose membranes (Amersham), which were blocked in 10% (wt/vol) nonfat milk in 50 mM Tris⋅HCl (pH 7.5), 150 mM sodium chloride, and 0.05% (wt/vol) Tween-20 for 30 min and further incubated with antibodies against immunopurified PFKFB3 (custom antibody as described in ref. 5), Cdh1 (CC43; Calbiochem), GLS1 (ab93434; Abcam), GAPDH (ab9485; Abcam), and β-actin (Sigma) overnight at 4 °C. After incubation with horseradish peroxidase-coupled secondary antibody (DAKO) for 1 h at room temperature, signal detection was performed by enhanced chemiluminescence (ECL; Amersham).
In Vitro Ubiquitination Studies.
PFKFB3, GLS1, and some of their constructs (PFKFB3 KENmut DSGmut and GLS1 KENmut D boxmut) were overexpressed in HeLa cells synchronized with DTB and nocodazole. The proteins were extracted with NE-PER Nuclear Protein Extraction Kit buffer (Promega) in the presence of phosphatase inhibitors (PhosphoSafe; Roche) at 8 h after release from nocodazole. The cytosolic protein fractions were subjected to in vitro ubiquitination studies with a HeLa conjugation kit (ubiquitination reaction mix) from Boston Biochem (K-915) following the manufacturer's instructions. Enriched nuclear extracts, obtained from synchronized HeLa cells at 4 h and 12 h after release from nocodazole using NE-PER buffer in the presence of phosphatase inhibitors, were used as sources of APC/C-Cdh1 and SCF-β-TrCP, respectively. Ubiquitinated forms of PFKFB3 and GLS1 were detected by immunoblotting, using the corresponding antibody, as described in Immunoblotting.
DNA Replication Analysis by Flow Cytometry.
FACS analysis was carried out using the method of Eward et al. (32) with slight modifications. Briefly, 1 × 106 cells were pelleted by centrifugation then fixed in 80% methanol at −20 °C for at least 2 h. Methanol was removed by centrifugation, and cells were resuspended in PBS containing 50 μg/mL propidium iodide and 50 μg/mL RNase A. Cell acquisition and analysis were carried out with the CyAn ADP flow cytometer (DAKO; Becton-Dickinson) by quantifying the populations in G1, S, and G2/M.
Statistical Analysis.
Data obtained under different conditions were compared by using Student's t test. Values stated are means ± SEM. Results were considered to be significantly different when P < 0.05.
Supplementary Material
Acknowledgments
We thank Annie Higgs for editorial help in the preparation of this manuscript. This work was supported by Wellcome Trust Grant 086729 (to S.M. and S.L.C.).
Footnotes
The authors declare no conflict of interest.
See Commentary on page 20857.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1117500108/-/DCSupplemental.
References
- 1.Visintin R, Prinz S, Amon A. CDC20 and CDH1: A family of substrate-specific activators of APC-dependent proteolysis. Science. 1997;278:460–463. doi: 10.1126/science.278.5337.460. [DOI] [PubMed] [Google Scholar]
- 2.Peters JM. The anaphase-promoting complex: Proteolysis in mitosis and beyond. Mol Cell. 2002;9:931–943. doi: 10.1016/s1097-2765(02)00540-3. [DOI] [PubMed] [Google Scholar]
- 3.Li M, Zhang P. The function of APC/CCdh1 in cell cycle and beyond. Cell Div. 2009;4:2. doi: 10.1186/1747-1028-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almeida A, Bolaños JP, Moncada S. E3 ubiquitin ligase APC/C-Cdh1 accounts for the Warburg effect by linking glycolysis to cell proliferation. Proc Natl Acad Sci USA. 2010;107:738–741. doi: 10.1073/pnas.0913668107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colombo SL, et al. Anaphase-promoting complex/cyclosome-Cdh1 coordinates glycolysis and glutaminolysis with transition to S phase in human T lymphocytes. Proc Natl Acad Sci USA. 2010;107:18868–18873. doi: 10.1073/pnas.1012362107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pardee AB. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci USA. 1974;71:1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tudzarova S, et al. Two ubiquitin ligases, APC/C-Cdh1 and SKP1-CUL1-F (SCF)-β-TrCP, sequentially regulate glycolysis during the cell cycle. Proc Natl Acad Sci USA. 2011;108:5278–5283. doi: 10.1073/pnas.1102247108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamano H, Tsurumi C, Gannon J, Hunt T. The role of the destruction box and its neighbouring lysine residues in cyclin B for anaphase ubiquitin-dependent proteolysis in fission yeast: Defining the D-box receptor. EMBO J. 1998;17:5670–5678. doi: 10.1093/emboj/17.19.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfleger CM, Kirschner MW. The KEN box: An APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 2000;14:655–665. [PMC free article] [PubMed] [Google Scholar]
- 10.Herrero-Mendez A, et al. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- 11.Gasteiger E, et al. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31:3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karamysheva Z, Diaz-Martinez LA, Crow SE, Li B, Yu H. Multiple anaphase-promoting complex/cyclosome degrons mediate the degradation of human Sgo1. J Biol Chem. 2009;284:1772–1780. doi: 10.1074/jbc.M807083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardozo T, Pagano M. The SCF ubiquitin ligase: Insights into a molecular machine. Nat Rev Mol Cell Biol. 2004;5:739–751. doi: 10.1038/nrm1471. [DOI] [PubMed] [Google Scholar]
- 14.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: The control of NF-κB activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 15.Nakayama KI, Nakayama K. Regulation of the cell cycle by SCF-type ubiquitin ligases. Semin Cell Dev Biol. 2005;16:323–333. doi: 10.1016/j.semcdb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Mayya V, et al. Quantitative phosphoproteomic analysis of T cell receptor signaling reveals system-wide modulation of protein-protein interactions. Sci Signal. 2009;2:ra46. doi: 10.1126/scisignal.2000007. [DOI] [PubMed] [Google Scholar]
- 17.Hampson LJ, Mackin P, Agius L. Stimulation of glycogen synthesis and inactivation of phosphorylase in hepatocytes by serotonergic mechanisms, and counter-regulation by atypical antipsychotic drugs. Diabetologia. 2007;50:1743–1751. doi: 10.1007/s00125-007-0696-y. [DOI] [PubMed] [Google Scholar]
- 18.Dean AM, Shiau AK, Koshland DE., Jr Determinants of performance in the isocitrate dehydrogenase of Escherichia coli. Protein Sci. 1996;5:341–347. doi: 10.1002/pro.5560050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hart M, et al. The F-box protein β-TrCP associates with phosphorylated β-catenin and regulates its activity in the cell. Curr Biol. 1999;9:207–210. doi: 10.1016/s0960-9822(99)80091-8. [DOI] [PubMed] [Google Scholar]
- 20.Skaar JR, Pagano M. Cdh1: A master G0/G1 regulator. Nat Cell Biol. 2008;10:755–757. doi: 10.1038/ncb0708-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersen BO, et al. Cell cycle- and cell growth-regulated proteolysis of mammalian CDC6 is dependent on APC-CDH1. Genes Dev. 2000;14:2330–2343. doi: 10.1101/gad.832500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reitzer LJ, Wice BM, Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem. 1979;254:2669–2676. [PubMed] [Google Scholar]
- 23.Melnykovych G, Bishop CF. Utilization of hexoses and synthesis of glycogen in two strains of HeLa cells. In Vitro. 1972;7:397–405. [PubMed] [Google Scholar]
- 24.DeBerardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: Metabolism and tumor cell growth. Curr Opin Genet Dev. 2008;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeBerardinis RJ, et al. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zielke HR, Ozand PT, Tildon JT, Sevdalian DA, Cornblath M. Reciprocal regulation of glucose and glutamine utilization by cultured human diploid fibroblasts. J Cell Physiol. 1978;95:41–48. doi: 10.1002/jcp.1040950106. [DOI] [PubMed] [Google Scholar]
- 27.Newsholme EA, Crabtree B, Ardawi MS. The role of high rates of glycolysis and glutamine utilization in rapidly dividing cells. Biosci Rep. 1985;5:393–400. doi: 10.1007/BF01116556. [DOI] [PubMed] [Google Scholar]
- 28.Mazurek S, Grimm H, Boschek CB, Vaupel P, Eigenbrodt E. Pyruvate kinase type M2: A crossroad in the tumor metabolome. Br J Nutr. 2002;87(Suppl 1):S23–S29. [PubMed] [Google Scholar]
- 29.Wellen KE, et al. The hexosamine biosynthetic pathway couples growth factor-induced glutamine uptake to glucose metabolism. Genes Dev. 2010;24:2784–2799. doi: 10.1101/gad.1985910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wellen KE, et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krude T, Jackman M, Pines J, Laskey RA. Cyclin/Cdk-dependent initiation of DNA replication in a human cell-free system. Cell. 1997;88:109–119. doi: 10.1016/s0092-8674(00)81863-2. [DOI] [PubMed] [Google Scholar]
- 32.Eward KL, et al. DNA replication licensing in somatic and germ cells. J Cell Sci. 2004;117:5875–5886. doi: 10.1242/jcs.01503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.