Abstract
The small GTPase Rheb is a positive upstream regulator of the target of rapamycin (TOR) complex 1 in mammalian cells and can bind directly to TOR complex 1. To identify the regions of the Rheb surface most critical for signaling to TOR complex 1, we created a set of 26 mutants wherein clusters of 1–5 putative solvent-exposed residues were changed to alanine, ultimately changing 65 residues distributed over the entire Rheb surface. The signaling function of these mutants was assessed by their ability, in comparison to wild type Rheb, to restore the phosphorylation of S6K1(Thr389) when expressed transiently in amino acid-deprived 293T cells. The major finding is that two mutants situated in the Rheb switch 2 segment, Y67A/I69A and I76A/D77A, exhibit a near total loss of function, whereas extensive replacement of the switch 1 segment and other surface residues with alanines causes relatively little disturbance of Rheb rescue of S6K1 from amino acid withdrawal. This is surprising in view of the minimal impact of guanyl nucleotide on Rheb switch 2 configuration. The loss of function Rheb switch 2 mutants are well expressed and exhibit partial agonist function in amino acid-replete cells. They are unimpaired in their ability to bind GTP or mammalian (m)TOR in vivo or in vitro, and the mTOR polypeptides retrieved with these inactive Rheb mutants exhibit kinase activity in vitro comparable with mTOR bound to wild type Rheb. We conclude that Rheb signaling to mTOR in vivo requires a Rheb switch 2-dependent interaction with an element other than the three known polypeptide components of TOR complex 1.
Rheb is a small GTPase in the Ras branch of the small GTPase superfamily (1), first identified as an mRNA whose abundance in rat brain increased rapidly after seizure induction and maneuvers that elicit long term potentiation (2). Rheb mRNA abundance in cultured mammalian cells is up-regulated by serum, growth factors, and UV irradiation. Two Rheb polypeptides, ~50% identical in amino acid sequence, are present in mammals, and Rheb polypeptides, ~40–50% identical in amino acid sequence to those in mammals, are present in several single cell eukaryotes, including Schizosaccharomyces pombe and Saccharomyces cerevisiae (3). A major physiologic function of Rheb was uncovered through screens in Drosophila for genes that affect cell size (4). Previous studies had shown that loss of function mutations of the genes encoding the subunits of the tuberous sclerosis complex polypeptides, TSC1 or TSC2, resulted in enlarged cells and constitutive activation of signaling through the rapamycin-sensitive TOR3 pathway (5–7). Mutational inactivation of Rheb was found to produce cells of small size, even in the absence of TSC function; reciprocally, overexpression of Rheb greatly increased cell size and stimulated TOR signaling in a rapamycin-sensitive manner (8–10). The ability of Rheb inactivation to reverse the large size phenotype of TSC deficiency suggested that Rheb acted downstream of the TSC and was perhaps the dominant effector of TSC on cell size. A satisfactory biochemical explanation for the genetic interactions observed between TSC and Rheb was provided by the finding that the TSC1/2 heterodimer is a GTPase activator for Rheb (11, 12), and Rheb is nearly completely GTP-charged in vivo in the absence of TSC.
To explore the mechanism by which Rheb promotes TOR signaling, we utilized an in vivo assay of Rheb activation of TOR signaling based on the ability of overexpressed recombinant Rheb to stimulate the rapamycin-sensitive phosphorylation of S6K1 at Thr389/412 in cells deprived of amino acids (13, 14). Withdrawal of amino acids or leucine alone causes a time-dependent dephosphorylation of S6K1(Thr389/412) (15). This phosphorylation is catalyzed directly by TOR complex 1 (16, 17) and requires the binding of wild type S6K1 to the TORC1 polypeptide raptor. In leucine-depleted cells S6K1(Thr389) phosphorylation is unresponsive to insulin, but can be restored by readdition of leucine or, in the absence of leucine, by overexpression of wild type Rheb (13, 14). Mutations in the Rheb switch 1 domain (e.g. T38M, I39K, and N41A) reduce or eliminate Rheb rescue of S6K1 phosphorylation, as do the Rheb mutations S20N or D60I, which eliminate the ability of Rheb to bind guanyl nucleotides. The ability of overexpressed wild type Rheb to restore TORC1 signaling appears to require the direct binding of Rheb-GTP to the TORC1 complex. Overexpressed wild type and mutant nucleotide-deficient Rhebs can each bind directly to TORC1; however, whereas the mTOR polypeptides bound to wild type Rheb exhibit kinase activity assayed in vitro, and mTOR polypeptides bound to the nucleotide-deficient Rheb mutants (that fail to restore S6K1 phosphorylation in amino acid-deficient cells) are entirely devoid of kinase activity in vitro (13). The importance of the direct binding of Rheb to TOR for TOR signaling is supported by the finding that some (I39K and N41A) but not all (T38M) Rheb loss of function Rheb switch 1 mutants show diminished retrieval of endogenous mTOR (13, 18); conversely, a mutant of S. pombe Rheb (K120R) selected for enhanced signaling in vivo exhibits a parallel enhancement of its association with endogenous SpTOR (19).
The binding of recombinant Rheb to endogenous and recombinant TOR exhibited several unexpected features. Most surprisingly, the association of Rheb with mTOR did not require Rheb guanyl nucleotide charging and was not enhanced (and was perhaps decreased) by charging with GTP rather than GDP (13). This behavior, essentially unique for the interaction of a Ras-like GTPase with a putative effector, was evident both during transient expression and in direct binding in vitro of the purified polypeptides. The binding of Rheb to mTOR was localized to mTOR-(2148–2300), which corresponds to the amino-terminal lobe of the mTOR catalytic domain. Surprisingly, Rheb was observed to also bind, in vivo and in vitro, to LST8 and to the carboxyl-terminal segment of raptor (13). Nevertheless, whereas the binding of Rheb to full-length mTOR, as well as the mTOR carboxyl-terminal fragment 2148–2549, is inhibited by withdrawal of the amino acid leucine, Rheb binding to LST8 or raptor is unaffected (14). Thus, the physiologic relevance of Rheb binding to the latter two TORC1 components is uncertain, especially as those polypeptides are both seven-bladed WD domain propellers, and their proper folding during transient expression is not assured.
A structure of the Rheb polypeptide bound to GTP and GDP was described recently by Yu et al. (20). The overall Rheb structures are most closely related to those of Ras and Rap1, and like those polypeptides, the Rheb switch 1 segment (amino acids 33–41) shows a large change in configuration between the GTP/GMPPNP-liganded state and the GDP-liganded polypeptide. This is consistent with the likely participation of the Rheb switch 1 segment in the GTP-dependent signaling function of Rheb, a conclusion supported by the finding that the Rheb switch 1 mutants T38A and I39K are greatly impaired in their ability to restore S6K1(Thr389/412) phosphorylation in amino acid-deprived cells. Yu et al. (20) also detected two significant differences between the structures of Rheb and Ras, one involving the residues in Rheb homologous to those in Ras concerned with GTP hydrolysis, and a second related to the structure of the switch 2 segment and the dependence of its conformation on the binding of GTP versus GDP. Whereas the Ras switch 2 contains a 10–11-residue α-helix, the Rheb switch 2 segment is primarily an extended loop that contains a very short helical stretch (amino acids 72–74) and is positioned differently in relation to the core structure as compared with the Ras switch 2. Moreover, whereas the Ras switch 2 segment exhibits a strong GTP-dependent reconfiguration, the Rheb switch 2 is only slightly reconfigured; the Rheb switch 2 loop amino-terminal to the short helix is relatively unaffected, whereas the short helix shows a modest displacement comparing the GTP-and GDP-liganded polypeptides. The availability of the Rheb structures enabled us to undertake a systematic mutagenesis of the residues on the Rheb surface, so as to inquire whether regions of Rheb apart from the switch 1 loop are important to the ability of Rheb to signal downstream to TORC1. The present results identify a set of amino acids in the Rheb switch 2 segment that exert a profound effect on the ability of Rheb to rescue S6K1 phosphorylation in amino acid-deprived cells.
EXPERIMENTAL PROCEDURES
Reagents
The QuikChange™ site-directed mutagenesis kit was from Stratagene. pEBG-p70S6k, pCMV5-FLAG-mTOR-(2148–2549), pCMV5-FLAG-LST8, and pcDNA1-FLAG-raptor-(1009–1335) were described previously (13). Anti-FLAG M2 antibody was purchased from Sigma, and anti-p70S6K(phospho-Thr389) was from Cell Signaling; anti-GST antibody was from Santa Cruz Biotechnology, Inc., and the GSH-Sepharose from GE Healthcare. GDP, GTP, and GTPγS were from Sigma. [32P]Orthophosphate was from PerkinElmer Life Sciences. D-PBS was from Invitrogen.
Generation of Rheb Mutants
Plasmids encoding all mutant versions of Rheb were generated with the QuikChange™ site-directed mutagenesis kit from Stratagene. Primers were designed to contain the desired mutations, and the mutagenesis was performed following the manufacturer’s instruction. Each mutant was constructed in both the pCMV5-FLAG and pEBG vectors. All mutants were verified by sequencing.
Cell Culture and Transfection
HEK293T cells were grown in Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with 10% fetal bovine serum (Sigma), 100 units/ml penicillin, and 0.1 mg/ml streptomycin in 10% CO2 at 37 °C. Transient transfection was performed by the lipofection method with Lipofectamine following the manufacturers’ instructions. Amino acid withdrawal procedures were described as before (14). In the experiments employing RNA interference to knockdown endogenous Rheb in HeLa cells (Fig. 3B), control siRNA or Rheb siRNA was transfected with Lipofectamine 2000. The Rheb siRNA (sequence UCAGUGUAGUUUGUUGUUUAA) targets Rheb 3′-untranslated region; the control siRNA is CACAACUCGCGUGCCGAGUUCAUAA. One day after siRNA transfection, cells were transfected with pCMV5-FLAG vector or pCMV5-FLAG-Rheb WT or mutants. Cells were harvested and analyzed by immunoblot 48 h after plasmid transfection.
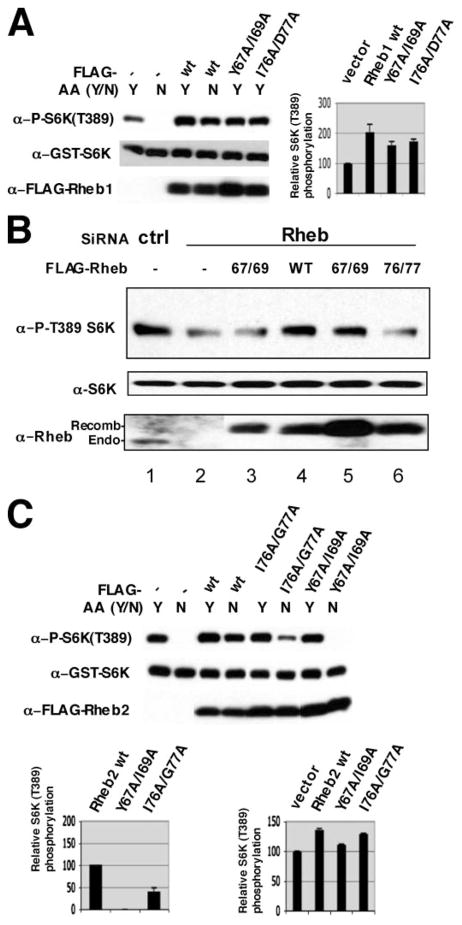
FIGURE 3. S6K(Thr389) phosphorylation induced by Rheb1 and Rheb2 wild type and switch 2 mutants in amino acid-replete and -deprived 293 cells.
A, comparison of the effects of Rheb1 wild type and Rheb1 switch 2 mutants (Y67A/I69A and I76A/D77A) on S6K(Thr389) phosphorylation in amino acid-replete and -deprived cells; the bar graph compares the ability of the Rheb1 variants to stimulate S6K(Thr(P)389) in replete cells. Transfections, amino acid withdrawal, and immunoblotting were done as described in Fig. 2A; for quantification of the response to Rheb in replete cells, the value of the S6K(Thr(P)389) immunoblot was estimated as in Fig. 2B, except that normalization for mutant Rheb polypeptide expression was applied only to the increment of S6K(Thr(P)389) above the vector control. The values are the mean of two experiments, and the error bar indicates the range. B, HeLa cells were transfected with control siRNA (lane 1) or Rheb1 siRNA (lanes 2– 6) on day 1. On day 2, the cells were transfected with pCMV5-FLAG (lanes 1 and 2) or pCMV5-FLAG Rheb WT or Rheb switch 2 mutants (as indicated). 48 h thereafter, the cells were harvested, and the phosphorylation of endogenous S6K1(Thr389) and the expression of S6K and Rheb1 were analyzed by immunoblotting. C, comparison of the effects of Rheb2 wild type and Rheb2 switch 2 mutants (Y67A/I69A and I76A/D77A) on S6K(Thr389) phosphorylation in amino acid-replete and -deprived cells; the bar graph on the left compares the ability of the Rheb2 variants to stimulate S6K(Thr(P)389) in amino acid-depleted cells; quantitation is as in Fig. 2B. The bar graph on the right quantifies these responses in amino acid-replete cells, and quantitation is as in A. The value for wild type Rheb2 is set at 100, and the mean and range of two experiments is shown.
Assays of Rheb Function
The assay of the ability of Rheb to signal to TOR complex 1 in vivo involved transient coexpression of FLAG-tagged wild type- or mutant-Rheb with an S6K1 reporter in 293T cells. Two hours prior to harvest, the medium was replaced with D-PBS. Extracts were prepared as in Ref. 13 and subjected to immunoblot for Rheb, S6K1 polypeptide, and S6K1(Thr(P)389). The dose of wild type Rheb chosen was 0.5 μg of DNA, which restores S6K1(Thr(P)389) to 50–70% of maximum. For assays of Rheb guanyl nucleotide binding in vitro, Rheb wild type and mutant polypeptides were transiently expressed as GST fusion proteins in HEK293T cells. Cells were extracted in ice-cold buffer (25 mM HEPES, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 0.25% sodium deoxycholate, 10% glycerol, 10 mM MgCl2, 1 mM EDTA, leupeptin (10 μg/ml), and aprotinin (10 mg/ml)), cleared by centrifugation, and incubated at 4 °C for 1 h with GSH-Sepharose beads. Adsorbed proteins were washed three times in extraction buffer and eluted with 10 mM reduced glutathione in 50 mM Tris, pH 8.
Guanine nucleotide binding was determined as described previously (21). GST fusion Rheb (1.5 μg) was incubated at 30 °C in binding buffer (20 mM Tris, pH 7.4, 50 mM NaCl, 0.1% Triton X-100, 1 mM dithiothreitol, 40 μg/ml bovine serum albumin, 1 mM EDTA, 1 mM MgCl2, and 2 μM [35S]GTPγS, or [3H]GDP (each at 2 Ci/mmol; PerkinElmer Life Sciences)). At the times indicated, ice-cold wash buffer (20 mM Tris, pH 7.5, 100 mM NaCl, 10 mM MgCl2, and 1 mM dithiothreitol) was added, and the samples were rapidly filtered through nitrocellulose filters and washed twice with this buffer. The amount of bound guanine nucleotide was measured by scintillation counting. Rheb guanyl nucleotide binding in vivo, protein-protein interactions during transient expression or in vitro, and the assays of mTOR kinase bound to recombinant GST-Rheb were performed as described in Ref. 13.
RESULTS
Generation of Rheb Mutants That Collectively Change All Surface Residues to Alanines
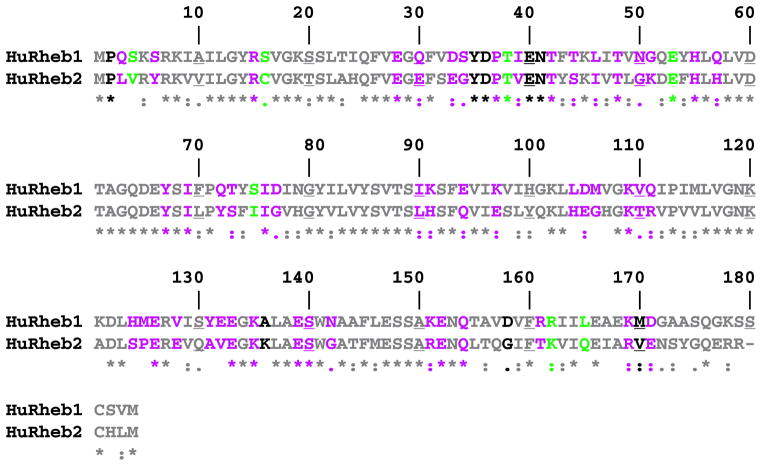
Based on the assumption that surface-exposed amino acid side chains are critical for the interaction of Rheb with its effectors, we undertook to change clusters of solvent-exposed side chains to methyl groups through mutation of contiguous surface residues to alanine. We inferred that the loss of function caused by mutation of residues in the switch I region (by homology, Rheb residues 31–43; see Refs. 13, 18, 20), indicates that, as with Ras (22), the switch 1 region is exposed on the Rheb surface in the GTP-liganded polypeptide in a manner that enables interaction with Rheb effectors. Moreover, we assumed that the residue side chains most highly exposed to solvent in the fully folded Rheb structure were likely to be less critical for proper Rheb folding as compared with buried residues. To identify Rheb residues appropriate for mutation, we examined the recently published human Rheb structure (complexed with either GTP or GDP) (20) with the DeepView program, available at the Swiss Protein Database. The function “accessible amino acids” identifies residues based on their solvent accessibility relative to the solvent accessibility of residue X in an extended pentapeptide GGXGG, whose solvent-accessible surface is set at 100%. Thus at an accessibility setting of 100%, none of the amino acids in the Rheb-GTP structure are considered to have a surface whose solvent accessibility is equal to the solvent accessibility of residue X. To identify probable Rheb surface residues, we therefore reduced the percent of surface accessibility required relative to residue X until the DeepView program identified most of the Rheb switch 1 residues as solvent-accessible; at an accessibility setting of 54%, Ile39 was the first Rheb switch 1 residue to be designated as solvent-accessible, and Asp36 and Gln30 became “accessible” at a setting of 44%. The switch 1 residue Asn41, whose mutation to alanine was shown previously to result in a partial loss of signaling to TOR complex 1 (13, 18), became solvent-accessible at a setting of 27%. We therefore chose a level of 25% of surface accessibility relative to residue X; at this setting, Rheb amino acids 33–37 and 39–42 are considered by the DeepView program to be solvent-exposed in the Rheb-GTP structure. Overall, 58 of the first 172 Rheb amino acids are solvent-exposed in Rheb-GTP, among which 50 are also exposed in the Rheb-GDP structure; of the eight residues solvent-exposed exclusively in Rheb-GTP, four are in the switch 1 segment. In addition, seven other residues are solvent-accessible only in Rheb-GDP (Fig. 1). The 58 putative solvent-exposed residues in Rheb-GTP were clustered and collectively changed to alanines through a total of 20 mutants that each change 1–5 amino acids (Table 1, class I). As regards the residues that are solvent-exposed only in Rheb-GDP, Ser16, Thr38, Glu53, and Ser75 were individually changed to alanines; Ser4 was converted to alanine together with the constitutive surface residues Gln3 and Ser6, and Arg162 and Leu165 were converted to alanine together with the buried residues Ile163/164 (Table 1, class II). All mutants were constructed based on two plasmids, pCMV5-FLAG-Rheb and pEBG-Rheb, allowing for expression of FLAG- or GST-tagged Rheb in mammalian cells, respectively. An alignment of human Rheb1 and Rheb2 and the residues that were mutated in Rheb1 are shown in Fig. 1. All Rheb mutants are listed in Table 1.
FIGURE 1. Sequence alignment of human Rheb1 and Rheb2.
An alignment of human Rheb1 and Rheb2 (3) by ClustalW is shown; every 10th residue is underlined. The colors indicate the solvent accessibility of each residue in the x-ray crystallographic structures of Rheb1 bound to either GTP or GDP (20), calculated by the solvent accessibility function of the DeepView program at a setting of 25% (Rheb2 is colored to facilitate comparison with Rheb1). As described under “Results,” the value of 25% was chosen so as to ensure solvent accessibility of all switch 1 residues whose mutation was previously shown to affect Rheb signaling to mTOR (13, 18). At this setting, gray residues are not solvent-exposed, purple residues are exposed in both Rheb-GTP and Rheb-GDP, black residues are exposed only in Rheb-GTP and green residues only in Rheb-GDP.
TABLE 1.
Rheb1 mutants generated in this study
Three classes of mutants were generated through site-directed mutagenesis. Class I contains mutants with Ala substitutions in residues that are solvent-accessible in both Rheb-GTP and Rheb-GDP or only in Rheb-GTP. Class II contains mutants with Ala substitutions in residues that are preferentially exposed to solvent in Rheb-GDP (see Fig. 1). Class III mutants are the human equivalent mutants of the hyperactive S. pombe Rheb mutants identified in Ref. 19.
| Class I | Class II | Class III | ||
|---|---|---|---|---|
| Arg15 | Y67I69 | H124M125E126V128 | Q3S4S6 | V17A |
| E28Q30 | Q72T73 | Y131E132E133K135 | Ser16 | V17G |
| D33S34Y35D36P37 | I76D77 | E139S140N142 | Thr38 | S21G |
| I39E40N41T42 | I90K91 | K151E152 | Glu53 | S21I |
| T44L46 | E94K97 | Q154D158R161 | Ser75 | K120R |
| T48N50G51 | L104D105M106 | K169M170D171 | R161R162I163I164 | N153S |
| H55Q57 | K109V110Q111 | N153T |
Of the 26 mutants, only the E94A/K97A mutant is poorly expressed after transient transfection in 293 cells. Further analysis showed that E94A or K97A individually has no effect on Rheb expression or S6K1 rescue; however, changing the Glu94 and Lys97 residues simultaneously (to Ala or Met) appears to have structural consequences that lead to low expression, presumably because of misfolding (data not shown). This mutant is excluded from further analysis. The other 25 mutants are well expressed (Fig. 2A).
FIGURE 2. Functional analysis of Rheb1 surface residue alanine mutants.
A, pCMV5-FLAG empty vector (Ctrl), FLAG-tagged Rheb1 WT (wt), or alanine mutants (mutated residues marked above each lane) were cotransfected with pEBG-p70 S6K in HEK293T cells. Forty hours thereafter, some plates were transferred to D-PBS (lanes 2–14, 16 –18, 20 –22, 24 –36, and 38 – 40), and all cells were harvested 2 h later. Aliquots of cell lysates containing equal amounts of protein were analyzed by immunoblot as indicated. B, quantitation of the ability mutant Rheb1 to restore p70 S6K(Thr389) phosphorylation in amino acid-deprived 293 cells. The ordinate indicates % S6K(Thr389) phosphorylation relative to that elicited by wild type Rheb1, the latter set at 100%. Quantitation was performed using NIH ImageQ software. The phospho-Thr389 S6K signal elicited by each Rheb1 variant was first normalized to the expression level of the Rheb1 polypeptide to obtain an S6K(Thr(P)389) value normalized for the modest variations in Rheb polypeptide expression; this value for each Rheb1 mutant was then divided by the normalized S6K(Thr(P)389) value of WT Rheb (set as 100) and multiplied by 100. The result shown for each mutant is the mean of three to five experiments ± 1 S.D.
Identification of Partial and Severe Loss-of-Function Rheb Mutants
Withdrawal of extracellular amino acids from 293 cells for 90–120 min leads to complete but reversible dephosphorylation of S6K1(Thr389/412) (15). Transient overexpression of wild type Rheb can, in a dose-dependent and rapamycin-sensitive manner, restore S6K1(Thr389) phosphorylation in amino acid-deprived cells, indicating that Rheb achieves this rescue through TORC1 (14). We therefore utilized this response to Rheb to assess the ability of the Rheb alanine mutants to signal to TORC1 in vivo. Inasmuch as we sought to identify strong loss of function mutants, a dose of wild type Rheb capable of ~50–70% of the maximal S6K1(Thr389) phosphorylation (14) was chosen for the comparison.
As illustrated by Fig. 2A, the comparison of the mutants to wild type Rheb was carried out only in experiments where the expression of the mutant Rhebs was equal or only slightly (i.e. less than 2-fold) greater than that of wild type Rheb, with the exception of the E94A/K97A mutant described above. The results of a series of three or more such comparisons are summarized in Fig. 2B; essentially, the vast majority of the Rheb alanine mutants are functionally “normal” in this assay, i.e. their ability to rescue S6K1(Thr389) phosphorylation from amino acid withdrawal is within 2 standard deviations of that exhibited by comparable expression of a half-maximal dose of wild type Rheb (whose effect is set arbitrarily in Fig. 2B to 100%). Two mutants, Y67A/I69A and I76A/D77A, show a severe defect, essentially a complete inability to rescue S6K1 from amino acid withdrawal. These inactivating mutations are located in the switch 2 region, flanking the short helix, amino acids 72–74. The surface residue mutants Q72/T73A and S75A are normal in their ability to restore S6K1(Thr(P)389), whereas the mutant Q72A/Y74A (not shown), which changes both a surface residue (Gln72) and an adjacent, solvent-inaccessible residue (Tyr74), is completely unable to rescue S6K1(Thr(P)389) despite robust expression.
When examined in amino acid-replete 293 cells, the Rheb(Y67A/I69A) and (I76A/D77A) mutants coexpressed with S6K1 do exhibit an ability to stimulate S6K1(Thr389) phosphorylation, with an efficacy approximately half that of wild type Rheb (Fig. 3A). Thus, these switch 2 mutants do not act in a dominant interfering manner but exhibit a reduced potency that is exaggerated by amino acid depletion. This contrasts with the inactive, nucleotide-deficient Rheb1 mutants S20N and D60I, which cause a modest inhibition of S6K1(Thr389) phosphorylation in replete medium (13, 23). To examine the responses to lower levels of recombinant Rheb in nutrient-replete cells, we reduced endogenous Rheb1 in replete HeLa cells using RNA oligonucleotides directed against Rheb1 3′-untranslated sequences; this results in a substantial reduction in endogenous Rheb1 polypeptide and a partial reduction in the phosphorylation of endogenous S6K1 on Thr389 (Fig. 3B, compare lane 1 with lane 2). On this background, modest overexpression of recombinant, wild type Rheb1 is sufficient to restore S6K1(Thr(P)389) to control levels (Fig. 3B, compare lane 4 to lane 1), whereas comparable overexpression of Rheb1 Y67A/I69A and I76A/D77A yields little or no restoration of S6K1(Thr(P)389) (Fig. 3B, compare lanes 3 and 6 with lane 4). Nevertheless, a higher level of Rheb1 Y67A/I69A expression does significantly restore S6K1(Thr(P)389) (Fig. 3B, compare lanes 5 and 3 with lane 1). Thus, although the ability of these Rheb switch 2 mutants to activate mTORC1 signaling is greatly reduced in amino acid-deprived cells (Fig. 2), they clearly exhibit partial agonist activity in nutrient-replete cells (Fig. 3, A and B). The reduced efficacy of these mutants in amino acid-deficient cells is likely due in part to the ability of amino acid depletion to reduce the interaction between Rheb and mTOR (14), an effect that is also observed with these switch 2 mutants (data not shown).
The Rheb1 mutant E28A/N30A shows a partial (~50%) loss of function that is statistically distinguishable from the response to wild type. E28A/N30A, located immediately amino-terminal to the switch I region, corresponds to nucleotide-invariant residues in Ras whose mutation impairs the transforming activity of Ras (24). Surprisingly however, two mutants that directly alter the Rheb switch I domain, Asp33-Ser34-Tyr35-Asp36-Pro37-Ala4, and Ile39-Glu40-Asn41-Thr42-Ala4, show no loss or only slight loss in their ability to rescue S6K1 from amino acid withdrawal-induced dephosphorylation. This is in contrast to the previous finding that the single mutation of the surface-exposed residue Asn41 reduces and I39K abolishes the Rheb rescue of S6K1 from dephosphorylation. It appears that the alanine mutations of the switch 1 segment are only modestly disruptive to the interaction of Rheb with its relevant effector(s) as reflected in this assay of Rheb function; the minimal effects of the switch 1 Ala mutants observed in these studies in comparison with the severe inhibition caused by the switch 2 Ala mutants emphasize the critical functional role of the switch 2 surface residues Y67A/I69A and I76A/D77A.
The Rheb1 residues Tyr67-Ile69 are conserved in Rheb2 (Fig. 1) as well as in Drosophila and S. pombe Rheb. In contrast, Rheb1 Ile76, although conserved in Rheb2 and S. pombe Rheb, is Met in Drosophila Rheb, and Rheb1 Asp77 is Gly in Rheb2 and S. pombe Rheb. We therefore inquired as to whether Ala mutations at these sites in Rheb2 affect its ability to signal to TORC1 (Fig. 3C). In amino acid-deprived cells, Rheb2 Y67A/I69A is completely unable to restore S6K(Thr389) phosphorylation, whereas Rheb2 I76A/G77A activity, although impaired, is less defective than the corresponding Rheb1 mutant. As with the Rheb1 switch 2 mutants, both Rheb2 switch 2 mutants continue to show partial activity in amino acid-replete cells.
We also examined the function of Rheb mutated at residues more solvent-exposed in the GDP-liganded than in the GTP-liganded structure (Fig. 2B). The T38A mutant exhibited a severe loss of function, as observed previously for T38M (13, 18). Thr38 participates in the coordination of Mg2+ and the γ-PO4 of GTP and undergoes a major flip inward in the Rheb-GTP structure (20). The Arg162-Ile163-Ile164-Leu165-Ala4 mutation causes a partial loss of function, accompanied by a marked upshift of the slower of the two polypeptide bands of transiently expressed Rheb seen on SDS-PAGE (Fig. 2A, lane 36). This mutant may undergo an aberrant carboxyl-terminal modification which, while having little effect on abundance, may underlie its diminished function. The mutants Asp33-Ser34-Tyr35-Asp36-Pro37/Ala5 (Fig. 2A, lane 8) and Thr48-Asn50-Gly51/Ala3 (Fig. 2A, lane 11), whose signaling is unimpaired, also exhibit a slowed mobility on SDS-PAGE, the basis for which is unknown. Gln3-Ser4-Ser6/Ala3 and the Ala mutants at Ser16, Glu53, and Ser75 each rescued S6K1(Thr389) phosphorylation in a manner indistinguishable from wild type Rheb.
Guanyl Nucleotide Binding of the Rheb Mutants
We sought next to characterize the biochemical properties of the Rheb mutants, seeking especially an explanation for the severe loss of function of the switch 2 mutants. We tested the ability of the loss of function mutants, Y67A/I69A and I76A/D77A, to bind to GTP. Wild type Rheb and the mutants were expressed in 293T cells, purified, and examined for their ability to bind [35S]GTPγS in vitro; the rate and extent of [35S]GTPγS binding by these mutants were similar to wild type Rheb (Fig. 4A). All of the other Rheb alanine mutants were also examined in this assay (data not shown); although a few of the Rheb alanine mutants exhibited rates of [35S]GTPγS binding in vitro that were reduced by up to 75% as compared with wild type Rheb, all nevertheless exhibited greater binding of [35S]GTPγS than the Rheb mutants S20N and D60I (the latter shown previously to have no ability to bind GTP in vitro or in vivo and to be inactive in rescue). Moreover, none of the Rheb mutants with moderately diminished [35S]GTPγS binding in vitro exhibited significantly reduced ability to rescue S6K1 from dephosphorylation during transient expression (Fig. 2, A and B), perhaps reflecting the level of expression chosen for the assay of their signaling ability.
FIGURE 4. Guanyl nucleotide binding of Rheb1 and Rheb1 switch 2 mutants.
A, GST-Rheb1 WT or mutants were expressed in and purified from HEK293T cells. In vitro binding to [35S]GTPγS was performed as described under “Experimental Procedures.” Bound guanyl nucleotide was quantitated by filtration and scintillation counting. B, in vivo guanyl nucleotide contents of Rheb1 WT, Y67A/I69A, I76A/D77A, and D60I mutants were determined as described under “Experimental Procedures” and in Ref. 13. After guanyl nucleotide extraction and separation by TLC, 32P comigrating with nonradio-active GDP and GTP was quantified by PhosphorImager. After subtraction of the 32P detected in the GST lanes, the total (32P-GDP + GTP) (in arbitrary PhosphorImager units) and the % of total (32P-GDP + GTP) as GTP are shown, ± 1 S.D. The GST immunoblot shows the relative amount of purified proteins.
We also examined the GTP/GDP content of the Y67A/I69A and I76A/D77A mutants during transient expression in 293T cells (Fig. 4B). Overexpression of Rheb was shown by Im et al. (25) to increase the fractional GTP charging compared with endogenous Rheb, perhaps because of exceeding the capacity of endogenous Rheb-GAP activity. In the current experiments transiently expressed GST-Rheb binds guanyl nucleotides at levels 5–20-fold over background, reflecting the expression level of the Rheb polypeptides, except for Rheb1 D60I, which binds no nucleotide as shown previously (13). The GST-RhebY67A/I69A and I76A/D77A mutants each exhibit robust guanyl nucleotide binding and a fractional binding of GTP that exceeds that of wild type Rheb expressed in parallel (Fig. 3B). Thus it is certain that the inability of these mutants to restore S6K1 phosphorylation during transient expression is not attributable to defective GTP charging.
Binding of Rheb Mutants to TORC1 Components
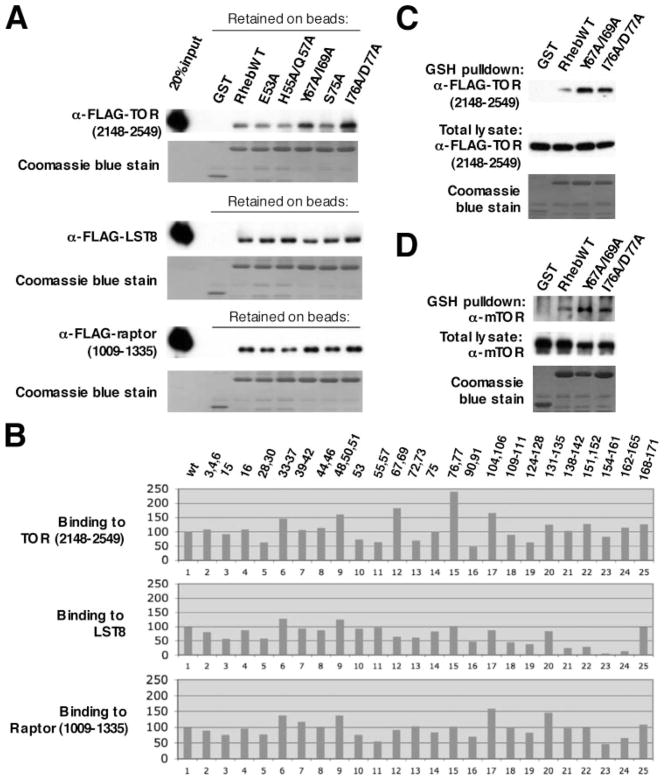
Recombinant Rheb can bind to TOR complex 1, both in vivo during transient expression and directly in vitro (13). Rheb binds to the amino-terminal lobe of the mTOR catalytic domain (2148–2300) and can also bind directly in vitro to mLST8 and to the carboxyl-terminal WD propeller segment of raptor. Several of the inactive Rheb mutants we examined previously were unimpaired in their binding to mTOR, and the nucleotide-deficient, inactive mutants S20N and D60I exhibited a substantially stronger binding to mTOR than wild type Rheb. Conversely, Tee et al. (18) reported that the hypoactive switch 1 mutant N41A exhibited a diminished ability to bind endogenous mTOR, and we found that the inactive Rheb switch 1 mutant I39K exhibits a greatly diminished ability to associate with endogenous mTOR during transient expression as well as a diminished association with the carboxyl-terminal fragment of mTOR, amino acids 2148–2549 (13), although its association with LST8 is similar to wild type Rheb.4 Thus nucleotide-deficient inactive Rheb mutants exhibit increased binding to mTOR, whereas some but not all (e.g. T38M) (13, 18) loss of function switch 1 mutants exhibit diminished binding to mTOR. We examined the ability of the Rheb alanine mutants to bind directly to the components of TOR complex 1; because of the difficulty in achieving uniform polypeptide expression during transient cotransfection, we examined the binding in vitro, using equal amounts of purified, immobilized GST-Rheb polypeptides, charged in vitro with GMPPNP, and the individual FLAG-tagged TORC1 component immunopurified after transient expression in 293T cells. As shown in Fig. 5A, purified mTOR-(2148–2549), mLST8, and raptor-(1009–1335) each binds specifically to purified GST-Rheb in vitro. The amount of each of these TORC1 components retained by each of the GST-Rheb-GMPPNP mutants, relative to that retained by wild type GST-Rheb-GMPPNP, is shown in Fig. 5B. The inactive mutants RhebY67A/I69A (Fig. 5B, lane 12) and I76A/D77A (Fig. 5B, lane 15) are unimpaired in their ability to bind to each of the TORC1 components; in fact these mutants bind ~2-fold greater amounts of mTOR-(2148–2549) than does wild type Rheb, and none of the other Rheb alanine mutants bind greater amounts of mTOR-(2148–2549) than do Rheb(Y67A/I69A) and -(I76A/D77A). By contrast the ability of Rheb(Y67A/I69A) and -(I76A/D77A) to bind mLST8 and raptor-(1009–1335) is indistinguishable from wild type Rheb. The modestly enhanced association of Rheb(Y67A/I69A) and (I76A/D77A) with the mTOR-(2148–2549) as compared with wild type Rheb is also evident when these components are transiently coexpressed (Fig. 5C), and is reflected as well in the ability of these Rheb variants to retrieve endogenous, full-length mTOR during transient expression (Fig. 5D). Thus the loss of function of these mutants is not attributable to a deficient interaction with TOR complex 1. The significance of the modestly enhanced association of Rheb(Y67A/I69A) and Rheb(I76A/D77A) with mTOR in relation to their loss of function is unclear; this behavior is similar to but much less pronounced than the enhanced binding of the inactive, nucleotide-deficient Rheb mutants S20N and D60I to the mTOR catalytic domain (e.g. see Fig. 6B) (13). Moreover, as observed with wild type Rheb and all Rheb mutants previously examined (14), the ability of Rheb(Y67A/I69A) and Rheb(I76A/D77A) to bind mTOR is greatly diminished by amino acid withdrawal (data not shown). This likely explains, in part, why the partial agonist activity of these mutants evident in nutrient-replete cells (Fig. 3, A and B) is not readily observed in amino acid-deficient cells (Fig. 2, A and B).
FIGURE 5. Binding of Rheb mutants to TORC1 components.
A, binding in vitro of GST-Rheb1 WT or alanine mutants to purified FLAG-mTOR-(2148 –2549), FLAG-LST8, and FLAG-raptor-(1009 –1335). Each FLAG-tagged polypeptide was expressed individually in HEK293T cells, immunopurified on immobilized anti-FLAG mono-clonal antibody, and eluted with FLAG peptide. GST, GST-Rheb1 WT, or mutants (marked on top of each lane) were also purified after transient expression, immobilized on Sepharose-GSH, and charged in vitro with GMP-PNP. Aliquots of each FLAG-tagged fragment were incubated in vitro with GSH-Sepharose-immobilized Rheb1 variants. After washing, the polypeptides retained on GSH-Sepharose were analyzed by anti-FLAG immunoblot (1st, 3rd, and 5th rows from top) and Coomassie Blue stain (2nd, 4th, and 6th rows from top). An aliquot representing 20% of the FLAG-polypeptide loaded with the GST proteins is in the 1st lane. B, relative binding in vitro of FLAG-mTOR-(2148 –2549), FLAG-LST8, and FLAG-raptor-(1009 –1335) to WT Rheb1 and all class I and II Rheb1 Ala mutants. The binding experiments were performed as described in A. For quantitation, the α-FLAG signal retained on beads in each lane was divided by the total GST-Rheb protein used in that binding, as shown by Coomassie Blue stain, to yield a normalized value that was divided by the normalized value for WT Rheb-GMPPNP (the latter set as 100) and multiplied by 100. C, binding of FLAG-TOR-(2148 –2549) to Rheb1 wild type and Rheb1 switch 2 mutants (Y67A/I69A and I76A/D77A) during transient coexpression. HEK293T cells were transfected with plasmids encoding FLAG-mTOR-(2148 –2549) and pEBG vector, pEBG-Rheb1 wild type, and Rheb1 switch 2 mutants (Y67A/I69A and I76A/D77A). Forty hours later, cells were harvested and lysates subjected to GSH-Sepharose affinity purification. The eluates and cell lysates were analyzed by anti-FLAG immunoblot and Coomassie Blue stain. D, the binding of GST-Rheb1 wild type and Rheb1 switch 2 mutants (Y67A/I69A and I76A/D77A) to endogenous mTOR. HEK293T cells were transfected with pEBG, pEBG-Rheb WT, or mutants. Forty hours later, cells were extracted, and the GST fusion proteins were purified on GSH-Sepharose. The GSH eluates and aliquots of the extract were analyzed by immunoblot for mTOR (top two panels). A Coomassie Blue stain of the GSH eluate is in the bottom panel.
FIGURE 6. mTOR bound to Rheb Y67A/I69A and I76A/D77A mutants exhibits normal kinase activity in vitro.
A, immunoblots of lysates from cells coexpressing HA-mTOR with GST, GST fusions to Rheb1 WT, or Rheb1(Y67A/I69A), -(I76A/D77A), and -(D60I) mutants. B, immunoblots of the GST proteins and copurifying HA-mTOR polypeptides after purification on GSH-Sepharose. Lanes 1–14 demonstrate the GST polypeptides as 1×, 2×, and 4× aliquots (bottom) and associated HA-mTOR (top). In the case of GST, only two aliquots were loaded. C, representative kinase assay of the GST-bound HA-mTOR polypeptides. After elution with GSH, aliquots containing equal amounts of HA-mTOR polypeptide, as shown by immunoblot (2nd panel from bottom), were assayed for kinase activity toward 4E-BP1 (lanes 1–5) or p70 S6K-(355–525) (lanes 6 –10). Substrate phosphorylation was estimated by immunoblot with anti-4E-BP (Thr(P)37/46) (lanes 1–5) or anti-p70 S6K(Thr(P)389); a representative immunoblot is shown above the corresponding bar graph, which in turn show a summary of two assays toward the 4E-BP1 substrate and four assays toward the p70 S6K substrate from two separate experiments. The results are expressed as a percentage (± 1 S.D. for the S6K substrate) of the mTOR kinase activity associated with WT Rheb (set to 100).
It should be noted that in no instance did a Rheb alanine mutant with retained signaling activity in vivo exhibit a substantial (e.g. >50%) decrease in binding to mTOR-(2148–2549) or to raptor-(1009–1330). Consequently, these results neither support nor refute the functional importance of the direct interaction between Rheb and mTOR or raptor for Rheb signaling in vivo. In contrast, there are several Rheb alanine mutants that are active in vivo but show little or no ability to bind mLST8 in vitro, most notably Rheb-(Q154A/D158A/R161A) (Fig. 5B, lane 23). This behavior indicates that the direct binding of Rheb to mLST8 in vitro is irrelevant to Rheb regulation of TORC1 in vivo and is likely of no physiologic significance.
mTOR Polypeptides Bound to the Inactive Rheb Mutants Y67A/I69A and I76A/D77A Retain Kinase Activity in Vitro
Although the inactive nucleotide-deficient Rheb mutants S20N and D60I bind endogenous and recombinant mTOR more strongly than does wild type Rheb, we observed previously that the mTOR polypeptides bound to S20N and D60I exhibit essentially no kinase activity when assayed in vitro using 4E-BP or a fragment of S6K1 (13). We therefore examined the kinase activity of mTOR polypeptides retrieved with the inactive Rheb mutants Y67A/I69A and I76A/D77A. As shown in Fig. 6, the mTOR polypeptides recovered with these two Rheb mutants exhibit protein kinase specific activity that is nearly equal to that of mTOR retrieved with wild type Rheb, and substantially greater than that of the mTOR bound to the RhebD60I (Fig. 6). Thus, the loss of signaling function shown by the RhebY67A/I69A and I76A/D77A mutants is not explained by an inability to enable mTOR to acquire protein kinase activity in vivo.
Mutations That Render S. pombe Rheb-hyperactive Do Not Alter the Ability of Human Rheb to Overcome the Dephosphorylation of S6K1(Thr389) Caused by Amino Acid Depletion
Loss of Rheb function renders S. pombe (as well as S. cerevisiae) hypersensitive to canavanine toxicity (3), presumably because of increased canavanine uptake, and Rheb overexpression confers canavanine resistance. Urano et al. (19) generated a library of random S. pombe rhb1 mutants and expressed these or wild type Rheb in a strain lacking the chromosomal rhb1 gene. They identified Rheb single amino acid mutants at four residues (V17A or V17K; S21G or S21I; K120R; N153S or N153T) that conferred resistance to canavanine toxicity not engendered by comparable expression of wild type Rheb; these Rheb mutants were presumed therefore to be hyperactive. They observed that a representative Rhb1 mutant at each of these four residues exhibited relatively normal GTP but greatly diminished GDP binding in vitro, and Rhb1K120R, expressed at near physiologic levels, exhibited greatly enhanced association with SpTOR after cell disruption. Impelled by these results, we examined the ability of Rheb V17A, S21G, and S21I, K120R and N153S and N153T to rescue S6K1 from amino acid-induced dephosphorylation; two doses of each mutant were compared with wild type Rheb, the latter at a dose chosen to enable 30% of maximal rescue, so as to readily detect hyper-active mutants. In no instance were these mutants found to be more potent than an equivalent amount of wild type Rheb in the restoration of S6K1(Thr(P)389) (Fig. 7). Whether this reflects qualitative differences between the present assay and that employed by Urano et al. (19) or simply the greater sensitivity of the latter is unclear.
FIGURE 7. Mutations that render S. pombe Rheb hyperactive do not alter the ability of human Rheb to rescue p70 S6K Thr389 from amino acid withdrawal-induced dephosphorylation.
A and B, 293T cells were transfected with pEBG-p70 S6K (lanes 1–14) and with pCMV5-FLAG vector (lanes 1 and 2) or with pCMV5-FLAG-Rheb1 WT (lanes 3 and 4; at a dose that allows ~30% rescue of p70 S6K Thr389 phosphorylation from amino acid withdrawal) or various Rheb1 mutants as indicated below each lane. Forty hours later, some plates were transferred to D-PBS (lanes 1 and 3–14), and all cells were harvested 2 h thereafter. The bar graph is a quantitation, as in Fig. 2B, of the anti-S6K(Thr(P)389) immunoblot results for the experiments shown in B. For the following mutants, the bar graph shows the average result from two samples: V17A, S21G, K120R, and N153T; for the following mutants, the bar graph represents the result from one sample: N153S (lane 11) and S21I (lane 14).
DISCUSSION
Previous work has established that the integrity of the Rheb switch 1 segment is critical for Rheb signaling to TOR complex 1 (13, 18). The primary conclusion of this analysis is that replacement of several surface-exposed amino acid side chains within the Rheb switch 2 segment with methyl groups results in a profound loss in the ability of Rheb to restore the signaling function of TOR complex 1 in amino acid-deprived cells, whereas alanine substitutions of surface residues in the Rheb switch 1 segment, including those shown previously to be critical for signaling, have a much more modest effect. Although an interaction of Ras-like GTPases with effectors through the GTPase switch 2 segments is not unexpected (reviewed in Ref. 22), the very strong inhibitory effect of these Rheb switch 2 mutations, as compared with comparable switch 1 mutations, is surprising, especially in view of the relative insensitivity of the configuration of the Rheb switch 2 segment to the binding of GTP versus GDP (20). The mechanism by which these switch 2 mutations disable the ability of Rheb to signal to mTOR is unknown; these inactive Rheb switch 2 mutants exhibit intact guanyl nucleotide binding in vitro and enhanced GTP charging in vivo. Their ability to bind directly to mTOR in vitro and in vivo is unimpaired, and probably modestly enhanced. Moreover, the mTOR polypeptides bound to the inactive switch 2 mutants exhibit a robust kinase activity in vitro. The features, as well as their robust expression after transient transfection, strongly support the likelihood that the overall configuration of these switch 2 mutants is well maintained. The findings of this study are highly dependent on the transfection assay used to estimate Rheb signaling activity. The validity of this assay as a monitor of the ability of Rheb to signal to TORC1 is based on the selective ability of Rheb but not Ras or Rap1, the small GTPases most similar to Rheb in primary sequence, to cause the restoration of S6K(Thr389) phosphorylation in amino acid-deprived cells, and on the ability of a brief treatment with rapamycin, a highly specific TORC1 inhibitor, to eliminate this response to Rheb. Nevertheless, the assay is only semi-quantitative and requires significantly supraphysiologic levels of Rheb expression (in part because amino acid deficiency inhibits the Rheb-mTOR interaction (14); in replete cells (e.g. Fig. 3B) recombinant Rheb is effective at more nearly physiologic levels). Moreover, a relatively high dose of wild type Rheb was chosen for comparison, biasing the assay toward the detection of strong loss of function mutations. Consequently, mutations that may cause modest loss of function, especially at physiologic levels of Rheb expression, were likely overlooked, e.g. the ala-nine substitutions in the Rheb switch 1 segment. Nevertheless, the present results establish the dominant importance of the Rheb switch 2 segment in signaling to TOR complex 1. Moreover, they show that the ability of Rheb-GTP to interact directly with TORC1 and support the configuration of TORC1 into a catalytically active moiety is not sufficient to enable Rheb stimulation of TORC1 signaling if the Rheb switch 2 loop is disrupted.
The lack of TORC1 response in vivo to the Rheb switch 2 mutants in amino acid-deprived cells is in one respect similar to the behavior of TORC1 in response to amino acid withdrawal per se. Thus, although amino acid depletion is capable of interrupting TORC1 signaling in vivo, this maneuver does not inhibit the kinase activity, assayed in vitro, of the TORC1 complexes immunoprecipitated directly after extraction from the amino acid-deficient cells (13, 15). We observed previously that amino acid withdrawal weakens the association of recombinant Rheb with mTOR, and suggested that such an inhibition of the binding of endogenous Rheb to endogenous mTOR may underlie the mechanism whereby amino acid depletion inhibits TORC1 signaling in vivo (14). Moreover, we hypothesized that the ability of recombinant Rheb to overcome amino acid depletion might be attributable to the ability of a large excess of recombinant Rheb-GTP to restore a productive Rheb-TORC1 interaction. The inability of the inactive Rheb switch 2 mutants to rescue TORC1, despite their robust GTP charging, direct binding to mTOR, and an apparent ability to enable TORC1 to achieve a catalytically active configuration indicates that there exists a further requirement for Rheb signaling to TORC1 in vivo. The Rheb switch 2 mutants, although greatly disabled in the amino acid-deficient milieu, exhibit partial activity when examined in amino acid-replete cells, stimulating S6K(Thr389) phosphorylation with roughly half the efficacy of wild type Rheb. The absence of a dominant-inhibitory phenotype when overexpressed suggests that their loss of function results from the loss of an interaction with an element necessary for effective signaling to TORC1, rather than a continued, possibly enhanced but nonproductive interaction with some critical element. The present data therefore suggest the existence of a Rheb partner apart from the three known polypeptide components of TORC1, which is necessary for TORC1 signaling whose association with Rheb is disabled by the switch 2 mutations. Whether this putative Rheb partner operates in physical proximity to TORC1 or further upstream is not known. Nevertheless, the requirement for accessory participants is now well appreciated in regard to Ras signaling. Thus Ras selection and/or regulation of its effectors may require one or more noncatalytic proteins that act as scaffolds or chaperones such as CNK or KSR (26), Sur-8 (27, 28), or galectin (29); these interact either with Ras, the effector, or both. Efforts to identify additional elements involved in the Rheb regulation of TORC1 are ongoing.
Footnotes
This work was supported by National Institutes of Health Grants DK17776 and CA73818.
The abbreviations used are: TOR, target of rapamycin; mTOR, mammalian TOR; GTPγS, guanosine 5′-3-O-(thio)triphosphate; D-PBS, Dulbecco’s phosphate-buffered saline; GST, glutathione S-transferase; siRNA, short interfering RNA; WT, wild type; GMPPNP, guanosine 5′-(β, γ-imino)triphosphate.
X. Long, Y. Lin, S. Ortiz-Vega, S. Busch, and J. Avruch, unpublished data.
References
- 1.Colicelli J. Sci STKE 2004. 2004;250:RE13. doi: 10.1126/stke.2502004re13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamagata K, Sanders LK, Kaufmann WE, Yee W, Barnes CA, Nathan D, Worley PF. J Biol Chem. 1994;269:16333–16339. [PubMed] [Google Scholar]
- 3.Aspuria PJ, Tamanoi F. Cell Signal. 2004;10:1105–1112. doi: 10.1016/j.cellsig.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Oldham S, Hafen E. Trends Cell Biol. 2003;2:79–85. doi: 10.1016/s0962-8924(02)00042-9. [DOI] [PubMed] [Google Scholar]
- 5.Gao X, Pan D. Genes Dev. 2001;15:1383–1392. doi: 10.1101/gad.901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Potter CJ, Huang HE, Xu T. Cell. 2001;105:357–368. doi: 10.1016/s0092-8674(01)00333-6. [DOI] [PubMed] [Google Scholar]
- 7.Tapon N, Ito N, Dickson BJ, Treisman JE, Hariharan IK. Cell. 2001;105:345–355. doi: 10.1016/s0092-8674(01)00332-4. [DOI] [PubMed] [Google Scholar]
- 8.Patel PH, Thapar N, Guo L, Martinez M, Maris J, Gau CL, Lengyel JA, Tamanoi F. J Cell Sci. 2003;116:3601–3610. doi: 10.1242/jcs.00661. [DOI] [PubMed] [Google Scholar]
- 9.Saucedo LJ, Gao X, Chiarelli DA, Li L, Pan D, Edgar BA. Nat Cell Biol. 2003;5:566–571. doi: 10.1038/ncb996. [DOI] [PubMed] [Google Scholar]
- 10.Stocker H, Radimerski T, Schindelholz B, Wittwer F, Belawat P, Daram P, Breuer S, Thomas G, Hafen E. Nat Cell Biol. 2003;5:559–566. doi: 10.1038/ncb995. [DOI] [PubMed] [Google Scholar]
- 11.Castro AF, Rebhun JF, Clark GJ, Quilliam LA. J Biol Chem. 2003;278:32493–32496. doi: 10.1074/jbc.C300226200. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Nat Cell Biol. 2003;5:578–581. doi: 10.1038/ncb999. [DOI] [PubMed] [Google Scholar]
- 13.Long XY, Lin, Ortiz-Vega S, Yonezawa K, Avruch J. Curr Biol. 2005;15:702–713. doi: 10.1016/j.cub.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 14.Long XS, Ortiz-Vega S, Lin Y, Avruch J. J Biol Chem. 2005;280:23433–23436. doi: 10.1074/jbc.C500169200. [DOI] [PubMed] [Google Scholar]
- 15.Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham C, Avruch J. J Biol Chem. 1998;273:14484–14494. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- 16.Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM. Proc Natl Acad Sci U S A. 1998;95:1432–1437. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isotani S, Hara K, Tokunaga C, Inoue H, Avruch J, Yonezawa K. J Biol Chem. 1999;274:34493–34498. doi: 10.1074/jbc.274.48.34493. [DOI] [PubMed] [Google Scholar]
- 18.Tee AR, Blenis J, Proud CG. FEBS Lett. 2005;579:4763–4768. doi: 10.1016/j.febslet.2005.07.054. [DOI] [PubMed] [Google Scholar]
- 19.Urano J, Comiso MJ, Guo L, Aspuria PJ, Denskin R, Tabancay AP, Jr, Kato-Stankiewicz J, Tamanoi F. Mol Microbiol. 2005;58:1074–1086. doi: 10.1111/j.1365-2958.2005.04877.x. [DOI] [PubMed] [Google Scholar]
- 20.Yu Y, Li S, Xu X, Li Y, Guan K, Arnold E, Ding J. J Biol Chem. 2005;280:17093–17100. doi: 10.1074/jbc.M501253200. [DOI] [PubMed] [Google Scholar]
- 21.Shao H, Kadono-Okuda K, Finlin BS, Andres DA. Arch Biochem Biophys. 1999;371:207–219. doi: 10.1006/abbi.1999.1448. [DOI] [PubMed] [Google Scholar]
- 22.Corbett KD, Alber T. Trends Biochem Sci. 2001;26:710–716. doi: 10.1016/s0968-0004(01)01974-0. [DOI] [PubMed] [Google Scholar]
- 23.Tabancay AP, Jr, Gau CL, Machado IM, Uhlmann EJ, Gutmann DH, Guo L, Tamanoi F. J Biol Chem. 2003;278:39921–39930. doi: 10.1074/jbc.M306553200. [DOI] [PubMed] [Google Scholar]
- 24.Marshall MS. Trends Biochem Sci. 1993;18:250–254. doi: 10.1016/0968-0004(93)90175-m. [DOI] [PubMed] [Google Scholar]
- 25.Im E, Von Linting FC, Chen J, Zhuang S, Qui W, Chowdhury S, Worley PF, Boss GR, Pilz RB. Oncogene. 2002;21:6356–6366. doi: 10.1038/sj.onc.1205792. [DOI] [PubMed] [Google Scholar]
- 26.Kolch W. Nat Rev Mol Cell Biol. 2005;6:827–837. doi: 10.1038/nrm1743. [DOI] [PubMed] [Google Scholar]
- 27.Li W, Han M, Guan KL. Genes Dev. 2000;14:895–900. [PMC free article] [PubMed] [Google Scholar]
- 28.Sieburth DS, Sun Q, Han M. Cell. 1998;94:119–130. doi: 10.1016/s0092-8674(00)81227-1. [DOI] [PubMed] [Google Scholar]
- 29.Ashery U, Yizhar O, Rotblat B, Elad-Sfadia G, Barkan B, Haklai R, Kloog Y. Cell Mol Neurobiol. 2006;26:471–495. doi: 10.1007/s10571-006-9059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]