Abstract
Background
Right ventricular hypertrophy (RVH) and RV failure contribute to morbidity and mortality in pulmonary arterial hypertension (PAH). The cause of RV dysfunction and the feasibility of therapeutically targeting the RV are uncertain. We hypothesized that RV dysfunction and electrical remodeling in RVH result, in part, from a glycolytic-shift in the myocyte, caused by activation of pyruvate dehydrogenase kinase (PDK).
Methods and Results
We studied 2 complementary rat models: RVH+PAH (induced by monocrotaline) and RVH+without PAH (induced by pulmonary artery banding, PAB). Monocrotaline-RVH reduced RV O2-consumption and enhanced glycolysis. RV 2-fluoro-2-deoxy-glucose uptake, Glut-1 expression and pyruvate dehydrogenase phosphorylation increased in monocrotaline-RVH. The RV monophasic action potential duration and QTc-interval were prolonged due to decreased expression of repolarizing voltage-gated K+ channels (Kv1.5, Kv4.2). In the RV working-heart model, the PDK inhibitor, dichloroacetate, acutely increased glucose oxidation and cardiac work in monocrotaline-RVH. Chronic dichloroacetate therapy improved RV repolarization and RV function in vivo and in the RV Langendorff model. In PAB-induced RVH, a similar reduction in cardiac output and glycolytic shift occurred and it too improved with dichloroacetate. In PAB-RVH the benefit of dichloroacetate on cardiac output was ~1/3 that in monocrotaline-RVH. The larger effects in monocrotaline-RVH likely reflect dichloroacetate’s dual metabolic benefits in that model: regression of vascular disease and direct effects on the RV.
Conclusion
Reduction in RV function and electrical remodeling in 2 models of RVH relevant to human disease (PAH and pulmonic stenosis) result, in part, from a PDK-mediated glycolytic shift in the RV. PDK inhibition partially restores RV function and regresses RVH by restoring RV repolarization and enhancing glucose oxidation. Recognition that a PDK-mediated metabolic shift contributes to contractile and ionic dysfunction in RVH offers insight into the pathophysiology and treatment of RVH.
Keywords: pulmonary artery banding, pulmonic stenosis, voltage gated potassium channels (Kv), Warburg hypothesis, mitochondrial metabolism
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a lethal syndrome caused by arterial obstruction related to excessive proliferation and impaired apoptosis of pulmonary artery smooth muscle cells (PASMC), endothelial dysfunction, inflammation and excessive vasoconstriction[1]. While pulmonary vascular disease is the obvious primary pathologic focus, right ventricular hypertrophy (RVH) and RV contractile dysfunction are major determinants of prognosis in PAH[2]. Some studies have shown that PAH induced by monocrotaline in animal models is associated with RV dysfunction[3, 4]. However, little is known about the specific mechanisms underlying RV dysfunction in PAH. Moreover, it is unclear whether therapies that target the RV (alone or in combination with the pulmonary vasculature) might be beneficial.
In RVH, prolongation of action potential duration (APD) and the QT interval corrected for heart rate (QTc) have been reported[5] and an accompanying downregulation of repolarizing Kv channels has been noted, namely Kv1.2, 1.5, 2.1, 4.2, and 4.3[6, 7]. QTc prolongation is also noted in humans with PAH[8]. However, the cause, consequences and reversibility of this electrical remodeling in RVH are unclear. Monophasic APD (MAPD) prolongation can promote arrhythmia[9] and increas cytosolic Ca2+ in cardiac myocytes[10]. The duration and positivity of the action potential’s plateau phase is also an important determinant of cardiac contractility[11]. Impaired early repolarization causes dysynchrony of the release of Ca2+ from the sarcoplasmic reticulum and decreases contractility; conversely therapies that restore normal repolarization are predicted to enhance contractility by improving excitation-contraction (EC) coupling[12]. A clue to the cause of electrical remodeling in RVH comes from positron emission tomography (PET) studies of small groups of PAH patients. Increased 2-fluoro-2-deoxy-D-glucose (FDG) uptake occurs in the RV in PAH[13, 14]. This suggests the RV may be glycolytic, moreover mild hyperoxia can improve experimental RVH, again suggesting that glucose oxidation may be impaired in RVH[15]. However, the cause of the increased FDG uptake and its relevance to the pathophysiology and treatment of RVH are unknown.
Interestingly, the PASMC[1] and endothelial cells[16] in PAH manifest a metabolic shift, characterized by enhanced glycolysis. Moreover, expression of Kv1.5 is downregulated in PAH PASMC, leading to membrane depolarization and calcium overload. Restoration of oxidative phosphorylation, using a prototypic pyruvate dehydrogenase kinase (PDK) inhibitor, dichloroacetate[17], decreases the severity of pulmonary hypertension and regresses RVH in multiple models of experimental PAH[1, 18]. It does so, in part, by restoring K+ channel expression/function, indicating a link between the metabolic state and the electrical remodeling in PASMC. In the vasculature, changes in metabolism appear to initiate upregulation of transcription factors that decrease Kv channel expression, including nuclear factor activating T- cells (NFAT)[19] and hypoxia inducible factor (HIF-1α)[1].
In left ventricular hypertrophy (LVH), glycolytic flux is increased and predominates over glucose oxidation[20]. Dichloroacetate doubles glucose oxidation and improves LV function in an LVH model challenged with ischemia-reperfusion[21]. Dichloroacetate also restores Ito in a rat myocardial infarction model[22]. These findings support a link between impaired aerobic metabolism, depressed Kv channel expression and impaired contractile function in the LV. The RV in PAH has similarities to hibernating LV myocardium in coronary artery disease patients. Recent data suggests that right coronary artery blood flow is reduced in PAH patients with severe RVH[2], suggesting chronic ischemia as a potential initiating stimulus for the proposed metabolic shift in severe RVH.
We used two different models of RVH to investigate whether the beneficial effects of PDK inhibition are secondary to reduced PAH, direct effects on the RV myocardium or a combination of the two. In the monocrotaline model there is an intrinsic coupling between severity of RVH and severity of the pulmonary vascular disease while pulmonary artery banding (PAB) results in RVH secondary to pure pressure overload. We simultaneously measured MAPD and RV contractility in an RV Langendorff model and assessed RV work and metabolism (using a dual isotope technique) in a working RV model to test the hypothesis that, in RVH, reduced RV function reflects two consequences of PDK activation, increased glycolysis and electrical remodeling that impairs early repolarization. A corollary of the hypothesis is that the PDK inhibitor dichloroacetate should restore repolarization and improve RV function by increasing glucose oxidation in both RVH models.
METHODS
The authors had full access to the data and take full responsibility for their integrity. All authors have read and agreed to the manuscript as written.
Experimental protocols
The University of Chicago Institutional Animal Care and Use Committee approved all protocols. In the monocrotaline model, adult male Sprague-Dawley rats weighing 260–280 g were randomized to the following groups: Control, Dichloroacetate, Monocrotaline, and Monocrotaline+Dichloroacetate. A single subcutaneous injection of monocrotaline (60 mg/kg, Sigma, St. Louis, MO) produced severe PAH and RVH at 1 month. The water for the Dichloroacetate and Monocrotaline+Dichloroacetate groups contained dichloroacetate 0.75 g/L, pH 7.0 (Sigma, St. Louis, MO) and rats consumed an estimated daily dose of 70 mg/kg. Dichloroacetate therapy started on day 10 post-monocrotaline, at which time PAH is established[18].
A pressure-overload RVH model was created by PAB. Penicillin G (600,000 U) was administered preoperatively. The rat was anesthetized with 3% isoflurane and then intubated. A median sternotomy was performed and the PA was dissected free from the aorta and left atrium. A silk suture was placed around the PA and a loose knot formed. A 16-gauge needle was inserted through the knot, parallel to PA. The suture was tied tightly and the needle was withdrawn, creating a stenosis equal to the needle’s diameter (~1.6 mm). After PAB, the thorax was closed. Pain relief was achieved using buprenorphine (15 µg/kg sc). The PAB protocol included four groups: Sham, Dichloroacetate, PAB, PAB+Dichloroacetate (0.75 g/L in the drinking water). Dichloroacetate therapy started on the same day as PAB surgery. The hearts were studied 7 weeks after PAB. Sham and Sham+DCA groups had gained 316±19 g and 318±17 g body weight (n=6–7, P>0.05) seven weeks after sham surgery. Although the increase of the body weight in PAB and PAB+DCA groups are less than the increase in Sham and Sham+DCA groups due to the surgery, PAB and PAB+Sham groups still had gained 268±16 g and 249±14 g body weight, respectively. There are no differences in weight gain between the two groups (n=6–7, P>0.05).
Echocardiography
Doppler, 2D and M-mode echo was applied to measure the PA acceleration time (PAAT), diastolic thickness and systolic thickness of the right ventricular free wall thickness (RVFW) (see methods in on-line Supplement).
Positron Emission Tomography (PET)
Glucose uptake was imaged in vivo in rats anesthetized using isoflurane gas anesthesia (2% in oxygen) using FDG as a radiotracer. PET Imaging was performed using a Triumph PET/SPECT/CT system (GE Healthcare, Northridge, CA). Rats fasted overnight and were given a sucrose load 30-minutes before FDG injection (20 × 45 mg sucrose pellets, Bilaney Consultants, NJ). Images were acquired 20 minutes after FDG injection. To provide anatomical references, the rat thorax was first imaged using micro-CT followed by a 60-minute PET data acquisition. Images are shown using a pseudo color map (red indicates high and green indicates low glucose uptake). Data were analyzed by manual segmentation of the LV and RV free walls of the 3D PET image dataset.
Catheterization
A Millar pressure catheter (Millar Instruments, Inc., Houston, TX) was used to measure RV systolic pressure (RVSP) and PA pressure (PAP) in the PAB model. The apex of RV was punctured with a 26-gauge needle and the catheter was threaded through it into the RV and then advanced across the PA banding site to measure PAP. Pressure was recorded by using a PowerLab data acquisition module (AD Instruments, Colorado Springs, CO).
RV Langendorff perfusion and measurement of MAPD
Rats were anesthetized with isoflurane and hearts were excised, perfused with a saline solution for 1–2 min at 25 °C and then mounted on a Langendorff perfusion apparatus for retrograde aortic perfusion with oxygenated Krebs-Henseleit buffer (95% O2+5% CO2) at a constant pressure of 75–85 cm H2O (37_C). The PO2 in the solution exceeded 560 mm Hg in all experiments. The AV node was ablated and hearts were paced at a cycle length of 200 ms. Hearts stabilized for 10–20 min and then right ventricular systolic pressure (RVSP) was measured, as described[18]. RV monophasic action potentials were recorded by placing Teflon-coated, 0.25 mm silver electrodes gently on the RV[23]. The signals were recorded using PowerLab and analyzed using Chart V 5.5.6. MAPD was measured as the time to achieve 20% (MAPD20) and 90% (MAPD90) of complete repolarization[23].
Measurement of RVH
RVH was measured as the ratio of RV/(LV+septum) weight.
O2-Consumption
RV respiration was measured by high-resolution respirometry (Oroboros, Innsbruck, Austria). Briefly, a section of RV (140 mg) was minced and added to a respirometry chamber containing 2ml saline at 37°C. The O2 concentration was recorded for 20 minutes and peak O2-consumption was measured using DatLab (Oroboros).
Simultaneous measurement of metabolism and function in the RV working heart model
In a separate set of experiments, an isolated working heart model was used to simultaneously measure metabolism and RV work (heart rate × RVSP). The heart was rapidly excised and subsequently mounted on an aortic perfusion cannula for about 10 min. The pulmonary veins, main PA and superior vena cava were isolated and cannulated. The inferior vena cava was isolated and ligated. To initiate flow to the RV and LV, perfusate was delivered to the superior vena cava and left atria cannulae at a constant preload (45 mm Hg and 115 mm Hg, respectively). Hearts worked against a 80 mm Hg aortic afterload, and a 45 mm Hg PA afterload. Cardiac output was measured with Transonic ultrasound flow probes in the afterload lines and cardiac work was calculated as the product of systolic pressure and cardiac output, as described[24]. Glycolysis and glucose oxidation were measured by perfusing hearts with Krebs–Henseleit buffer containing 11 mM [5-3H/U-14C] glucose, 0.5 mM lactate, 1.2 mM palmitate, 3% albumin, and 100 U/ml insulin, as previously described[25]. The effect of dichloroacetate on glucose oxidation and cardiac function was evaluated by quantitative collection of 3H2O (glycolysis) and 14CO2 (glucose oxidation), at 10 minute intervals after adding 1 mM dichloroacetate to the perfusate.
Statistics
Values were expressed as mean±SEM. The differences among the four groups were assessed by one-way ANOVA with post hoc testing using the Fisher probable least significant differences test. A paired t-test was used for assessing the effect of dichloroacetate at baseline versus 40 minutes of perfusion. A value of P<0.05 was considered statistically significant.
Electrocardiograms, qRT-PCR, immunofluorescence, immunoblotting, histology, thermodilution cardiac output
RESULTS
Hemodynamics, Histology and Cardiac function
Four weeks after monocrotaline, pulmonary hypertension was evident. The PAAT was shortened in Monocrotaline vs Control rats and was improved in the Dichloroacetate+Monocrotaline group (12.4±0.3, 35.0±0.7 vs 20.6±2.7 ms, respectively; n=6–12/group, P<0.001, Fig. 1A). Monocrotaline increased the RV/(LV+Septum) weight ratio versus control and this was reduced in the Dichloroacetate+Monocrotaline group (0.61±0.04, 0.27±0.01 vs 0.39±0.04, respectively n=10–11, P<0.05) (Fig. 1B). Dichloroacetate also reversed monocrotaline-induced hypertrophy of individual RV myocytes on histology (Fig. 1C). Increased RVFW thickness in Monocrotaline vs Control was also reduced in the Dichloroacetate+Monocrotaline group (1.67±0.06, 0.95±0.02 vs 1.37±0.10 mm, respectively; n=6–13, P<0.001, Fig. 1D). RV systolic function was reduced in Monocrotaline versus control rats, suggesting impaired RV contractility, and RVFW systolic shortening was significantly improved by dichloroacetate (85±10%, 19±10% vs 65±14%, respectively, n=3, P<0.05, Fig. 1E). In the Langendorff model, developed RVSP was significantly elevated in Monocrotaline vs Control and was decreased in the Dichloroacetate+Monocrotaline group (63±7, 31±3, 43±3 mm Hg, respectively, n=8–9, P<0.05, Fig 1F). In vivo, thermodilution CO was reduced in Monocrotaline vs Control (58.3±4.9 vs 154.2±16.2 ml/min, n=6, P<0.001, Fig. 1G) and this improved in the Dichloroacetate+Monocrotaline group to 124.6±15.7 ml/min, n=6, P<0.005). Dichloroacetate did not cause hemodynamic or histological changes in control rats (Fig. 1).
Figure 1. Dichloroacetate reduces RVH caused by Monocrotaline and improves cardiac function.
The upper panel shows the chronic regression protocol of the administration of monocrotaline and dichloroacetate. A. Representative and mean data showing that dichloroacetate (DCA) lengthens PAAT (lowers PAP) in monocrotaline-induced RVH. B. The RVH is reduced by dichloroacetate. C. H&E staining showing that dichloroacetate decreases the hypertrophy of RV myocytes. D. Increased right ventricular free wall (RVFW) thickness is reduced by DCA. E. The impaired RVFW systolic thickening in RVH is increased by DCA. F. Representative traces and mean values showing dichloroacetate reduces RVSP in RVH in the RV Langendorff model. G. DCA restores cardiac output in vivo in the MCT+DCA group.
CTR, Control group; DCA, Dichloroacetate group; MCT, Monocrotaline group; MCT+DCA, Monocrotaline+Dichloroacetate group.
Metabolic changes in RVH
In the RV working heart model, cardiac work tended to increase during a 40-minute period of observation. However, the only significant increase in cardiac work occurred in Monocrotaline hearts that received dichloroacetate (1 mM) in the perfusate (n=4–5, p=0.05) (Figure 2A).
Figure 2. Dichloroacetate acutely improves cardiac function and increases glucose oxidation in RVH in a working heart model.
A Cardiac work increased 40 minutes after addition of DCA to the perfusate, only in the RVH group. B. Addition of 1 mM DCA to the perfusate increased glucose oxidation 4-fold in monocrotaline hearts. C. Glycolysis increases in RVH.
Basal rates of glucose oxidation did not differ among the groups (Fig. 2B). However glycolysis rates were increased in Monocrotaline versus Control (4025±611 vs 1872±430 nmol/min*g dry wt, n=5, respectively. P<0.05. Fig. 2C). Addition of 1 mM dichloroacetate to the perfusate increased glucose oxidation in Monocrotaline RVH from 772±323 to 3426±312 nmol/min*g dry weight (n=5, P<0.001, Fig. 2B), with no change in glycolytic rates (Fig. 2C).
RV O2-consumption was reduced in Monocrotaline vs Control and this was restored in Monocrotaline+Dichloroacetate (148±27, 277±27 vs 230±22 pmol/sec*ml, respectively, n=6–7, P<0.05, Fig. 3A&B). Consistent with the increased glycolysis, FDG uptake was elevated in RV of monocrotaline rats (Fig. 3C). The RV/LV FDG uptake was increased in Monocrotaline vs Control (1.50±0.41 vs 0.46±0.03, n=7–9, P<0.05, Fig. 3D). Dichloroacetate tended to reduce FDG uptake ratio to 1.19±0.34 (n=11, P=NS)
Figure 3. Reduced O2-consumption and increased FDG uptake in RVH.
A and B. Representative traces and mean data showing DCA reverses the depressed O2-consumption in RVH. C and D. Representative images and mean data showing increased FDG uptake in the RV in RVH on PET scans.
Immnunostaining showed qualitatively greater expression of the cardiac glucose transporter Glut1[26] in monocrotaline-induced RVH, both in the cytosol and at the plasma membrane (where it colocalized with dystrophin). Glut1 expression was decreased toward normal in the Monocrotaline+Dichloroacetate group (Fig. 4A). Consistent with the immunofluorescence, Monocrotaline RVH was accompanied by a 3-fold increase expression of Glut1 mRNA (n=6, respectively, P<0.001, Fig. 4B). Dichloroacetate reduced Glut1 mRNA by 43% (n=6, P<0.05 vs Monocrotaline). Glut1 protein expression was also increased in Monocrotaline group and decreased in the Monocrotaline+Dichloroacetate group (n=4/group; P<0.05, Fig. 4C).
Figure 4. Increased Glut1 expression and PDH phosphorylation in RVH are reduced by dichloroacetate.
A. Immunostaining showing that the increased Glut1 expression in RV myocytes in RVH is reduced by DCA. The merge of the staining of Glut1 (green) and dystrophin (red) in RV shows that more Glut1 is expressed at the myocyte membrane in RVH. B and C, Glut1 mRNA and protein levels are significantly increased in RVH and DCA normalizes expression. D. Increased phosphorylation of PDH in RVH is reduced by dichloroacetate.
To confirm whether the effect of dichloroacetate was mediated via PDK inhibition, we measure PDH phosphorylation. Phosphorylated PDH increased 2.5-fold in the Monocrotaline group and this decreased in Monocrotaline+Dichloroacetate group (n=7–8, P<0.05, Fig. 4D). Dichloroacetate did not change Glut1 and PDH expression in control rats (Data not shown).
Expression of both Glut1 and phosphorylated PDH did not change in LV in either Monocrotaline or Monocrotaline+Dichloroacetate groups (See Supplemental Fig. 1).
Electrical remodeling in RVH in the Monocrotaline model
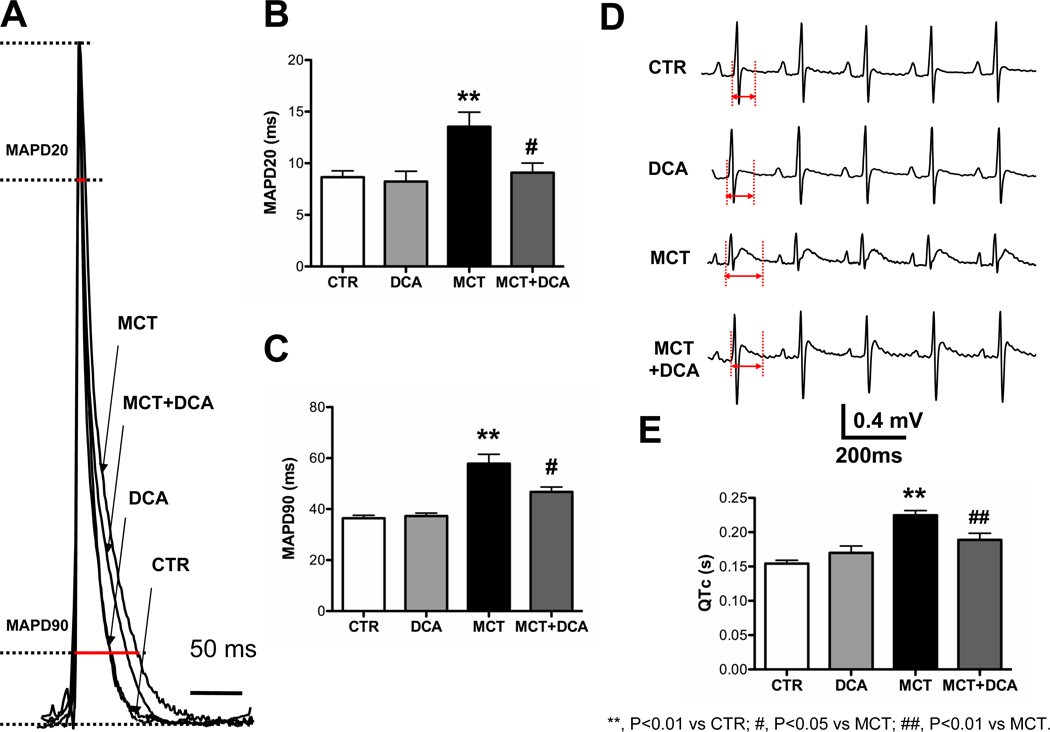
The time to achieve 20%and 90% of complete repolarization (MAPD20 and MAPD90) in the RV were significantly increased in Monocrotaline vs Control (Fig. 5A, B&C). Dichloroacetate shortened MAPD20 nearly to the Control levels. To determine whether dichloroacetate acutely changes MAPD, 1 mM dichloroacetate was added to the perfusate. MAPD did not change within 50 min (n=2, P=NS, see Supplemental Fig. 2), suggesting that shortened MAPD in Monocrotaline+Dichloroacetate group is more likely due to the documented re-expression of Kv channels following chronic in vivo dichloroacetate treatment (Fig. 6) than to an acute effect of dichloroacetate on channel activity.
Figure 5. Dichloroacetate shortens prolonged MAP duration and QTc in RVH.
A, B and C, Representative traces and mean data showing MAPD20 and MAPD90 are significantly prolonged in the MCT group vs CTR and that repolarization is improved by DCA. D and E, Representative traces and mean data of lead II surface ECG. DCA reduces the QTc prolongation in RVH. Red highlighted arrows indicate QTc intervals.
Figure 6. Repolarizing Kv channels that are downregulated in RVH are partially restored by dichloroacetate.
A, B and C, Kv1.2, Kv1.5 and Kv4.2 are significantly downregulated in RVH and DCA partially reverses this downregulation. D, E and F, Immunoblotting showing the downregulation of Kv1.2, Kv1.5 and Kv4.2 by monocrotaline. Kv1.5 expression in RH is enhanced by DCA.
Consistent with the prolongation of MAPD, the QTc was prolonged in Monocrotaline vs Control groups and was normalized in the Monocrotaline+Dichloroacetate group (n=6, P<0.05, Fig. 5D&E). Dichloroacetate had no effect on MAPD or QTc interval in normal rats (Fig. 5).
Expression of Kv1.2, Kv1.5 and Kv4.2 channel mRNA in the RV was downregulated in Monocrotaline vs Control groups (n=6, P<0.01, Fig. 6 A–C) and was partially restored by dichloroacetate. Kv1.4 and Kv2.1 mRNA did not change among the groups (n=4, P>0.05, Supplemental Fig. 3). At the protein level, Kv1.2, Kv1.5 and Kv4.2 expression was also significantly reduced in Monocrotaline vs Control groups (n=3–7, P<0.05, Fig. 6D, E&F). The expression of Kv1.5 protein was significantly increased in the Monocrotaline+Dichloroacetate group (n=7, respectively, P<0.05, Fig. 6E).
RVH induced by pulmonary artery banding
In order to distinguish whether dichloroacetate’s effects on RV function and hypertrophy reflected direct effects on the RV, independent of effects on the pulmonary vasculature, we used the PAB model. Histological study 7 weeks following PAB, showed RV myocyte hypertrophy in the PAB group, and this was partially reversed by concomitant dichloroacetate therapy (Fig 7a). The RVSP in RVH induced by PAB was reduced by dichloroacetate (23±2, 57±5 and 45±4 mm Hg, respectively, n=3–4, P<0.05, Fig 7B). Likewise, the peak pressure gradient between the pulmonary artery and RV pressure was significantly reduced by dichloroacetate (Fig 7B). Dichloroacetate improved RVFW systolic thickening (Control: 74±8%; PAB: 30±3%; PAB+Dichloroacetate: 54±6%, n=3–14, P<0.05, Fig. 7C). The CO, which was reduced in PAB group versus control, was partially restored in the PAB+Dichloroacetate group (177±11, 108±10 and 135±10 ml/min, respectively, n=4–9, P< 0.05, Fig 7D).
Figure 7. Dichloroacetate reverses RVH induced by pulmonary artery banding and improves cardiac function.
A. H&E staining and mean data of cell size show DCA reduces cell size in RVH. B representative traces showing the gradient across the stenosis in the PAB model. Mean±SEM of hemodynamic data in this model. C. The reduced RVFW systolic thickening in PAB is improved by DCA. D. Reduced cardiac output, measured by thermodilution, is improved by DCA.
Similar to the monocrotaline model, hypertrophied RV myocytes in the PAB model had greater Glut1 expression than control RV myocytes and chronic dichloroacetate therapy normalized both RV myocyte size and Glut1 expression (Fig. 8A).
Figure 8. Dichloroacetate reverses metabolic changes in RVH induced by pulmonary artery banding.
A. Immunostaining of Glut1 shows that the increased Glut1 expression at myocytes membranes is reduced by DCA. B. DCA reverses the impaired oxygen consumption in RVH. C. Dichloroacetate causes a greater relative increase in CO in the Monocrotaline versus the PAB Model. D. Proposed mechanism of hibernating RV myocardium in RVH. The activation of PDK in RVH reduces the coupling of glycolysis to glucose oxidation. These metabolic changes are (in part) rapidly reversible by dichloroacetate, which improves contractility by restoring normal RV energetics. PDK activation also inhibits the expression of Kv channels, which prolongs APD. Prolonged APD may also contribute to impairment of RV function in RVH.
The reduced RV O2-consumption in PAB relative to Control was restored in the PAB+Dichloroacetate group (296±23, 208±19 vs 309±33 pmol/sec*ml, n=6–7, P < 0.05, Fig. 8B), consistent with a dichloroacetate-mediated increase in glucose oxidation.
DISCUSSION
RV function is a major determinant of functional class and survival in pulmonary hypertension[2], heart failure and many forms of congenital heart disease. In this study we show that the hypertrophied RV undergoes a metabolic shift resulting in decreased RV function (Fig. 1E&G). Furthermore, we also demonstrated that the hypertrophied RV is associated with “electrical remodeling”, which we define as the changes in cardiac depolarization or repolarization which occur in disease as a consequence of altered expression or function of ion channels in the cardiac myocyte (Fig. 5 and Fig. 6). Specifically, there is increased glycolysis in RVH (Fig. 2C) as evidence by increased FDG uptake on PET scans in vivo (Fig. 3 C&D) and elevated Glut1 expression with an increased PDH phosphorylation (Fig. 4). Likewise, RVH and RV dysfunction induced by PAB are also associated with the reduced oxidative metabolism and increased Glut1 expression (Fig. 7 and Fig. 8). In an RV working heart model we show that enhancing glucose oxidation improves RV function in RVH (Fig. 2). Dichloroacetate accomplished this increase in glucose oxidation by reversing the pathological phosphorylation of PDH that occurs in RVH (Fig. 4D and Fig. 8B). Interestingly, dichloroacetate did not decrease glycolysis in RVH (Fig 2C). This may be related to the large increase in glucose oxidation seen in the dichloroacetate-treated hearts, which would result in a lowering of cytosolic pyruvate. A decrease in pyruvate, which is an end product of glycolysis, would stimulate a homeostatic increase in glycolysis to maintain glycolytic flux. Regardless, the large increase in glucose oxidation with dichloroacetate resulted in an improved coupling between glycolysis and glucose oxidation (Fig 2B and Fig 2C). In addition, the electrical remodeling is largely reversed by dichloroacetate (Fig. 5 and Fig. 6), indicating that, like the cardiac dysfunction, the electrical remodeling is metabolically mediated.
While prior research has shown downregulation of Kv channels in experimental RVH[5, 27, 28] and two observational studies have identified enhanced FDG uptake in the RV of patients with pulmonary hypertension[13, 14], our report is the first to link these metabolic and ionic abnormalities by identifying PDK activation as a reversible cause of both abnormalities. A major strength of this research is the use of two models of RVH-one caused by pulmonary hypertension (analogous to human PAH) and one which is caused by pulmonary artery banding (a model of pulmonic stenosis) in the absence of pulmonary vascular disease.
We have previously shown that there is a metabolic shift in the pulmonary vasculature in PAH and marked improvements in PAH can be seen when PDK is inhibited by dichloroacetate. However, since observational studies had reported increased glucose uptake in the RV of PAH patients[13, 14], we hypothesized that the hypertrophied RV myocardium could also exhibit a metabolic shift similar to what we had seen in the pulmonary vasculature[18]. We therefore comprehensively studied the metabolism of the hypertrophied RV.
A key finding of our study is the documentation of increased glycolysis in RVH (Fig. 2C). These metabolic measurements are reinforced by the findings of increased expression of Glut-1 (Fig. 4 B&C and Fig. 8A) and FDG uptake (Fig. 3C&D), reminiscent of hibernating myocardium seen in patients with chronic left ventricular ischemia due to coronary artery disease. Increased expression of glut1 is associated with a tendency of this transporter protein to localize to the plasmalemma (indicating that it is active) (Fig. 4A and Fig. 8A).
This study offers 3 new observations that have mechanistic and therapeutic relevance to RVH and which are consistent with the interpretation that the glycolytic metabolic shift is causally related to the changes in RV function and electrophysiology. We show that the metabolic abnormality in RVH:
Contributes substantially to both the RV dysfunction and the electrical remodeling.
Reflects PDK activation, PDH phosphorylation and a resulting increase in glycolysis.
Is reversible and significantly ameliorated by the PDK inhibitor, dichloroacetate.
There is precedent for the benefits of dichloroacetate on RV function. Nagendran et al. used a Langendorff perfusion system to show improved right ventricular inotropy when rats with MCT-induced RVH are treated with dichloroacetate [29]. They showed that dichloroacetate ”reversed both the phenylephrine-induced mitochondrial hyperpolarization and nuclear factor of activated T lymphocytes (NFAT) activation.” They did not measure RV function in vivo or directly measure RV metabolism. Thus while our study is consistent with theirs (suggesting a benefit for DCA in RVH), the two studies examine different mechanisms. Their work suggest a potential mechanism for the intersection between metabolism and electrical remodeling, in that changes in metabolism may ultimately lead to altered cardiac repolarization through a transcriptional mechanism that is regulated by NFAT.
The use of the PAB model, which induces RVH and RV dysfunction independent of pulmonary vascular disease, allowed identification of direct beneficial effects of dichloroacetate on the RV. The observation that dichloroacetate was also beneficial in the PAB model (albeit less so than in the monocrotaline model), indicates that PDK inhibition by dichloroacetate can indeed improve RV function and reduce RVH by directly acting on the RV. In the monocrotaline model dichloroacetate caused a 43% increase in CO; whereas in PAB dichloroacetate increased CO by a smaller amount (16%, Fig. 8C). It is not surprising that dichloroacetate has a larger benefit on RV function and RVH in the monocrotaline-PAH model since it exerts two complementary benefits in this model (reducing afterload through remodeling of the pulmonary vasculature and directly acting on the RV). Whereas, in the PAB model, the RV benefits are exclusively mediated via direct improvement in RV function, independent of any reduction of PA stenosis or pulmonary vascular resistance.
A number of technical innovations make our conclusion robust. The use of an RV Langendorff model (Fig. 1F) demonstrated the positive effects of dichloroacetate on RV function, independent of afterload, and also allowed simultaneous measurement of electrical function. The use of an RV working heart model and the dual isotope technique (Fig. 2) allowed simultaneous measurement of RV work and metabolism in response to dichloroacetate (at constant load). These models showed the increased glycolysis in monocrotaline-induced RVH is associated with reduced RV function and show that dichloroacetate’s ability to increase contractility by (i.e. increased RV work at a constant load-Fig. 2) is associated with increasing glucose oxidation. Finally the use of high fidelity catheterization and PET scanning confirmed that the hemodynamic and biochemical changes detected in isolated organs/tissues reflected the physiology in vivo.
Dichloroacetate improves metabolism in hypertrophied hearts
Our findings suggest that the hypertrophied RV is an example of hibernation, meaning the RV is viable but its function is depressed. Depressed RV function in both models results in part from PDH inhibition, caused by PDK-mediated phosphorylation. The metabolic shift to glycolysis appears to be responsible for much of the observed RV dysfunction and neither the metabolic or functional impairment occur in the LV (Fig. 3C and supplemental Fig. 1).
PDK is a key regulator of glucose oxidation. Activated PDK phosphorylates and inhibits PDH (Fig. 4D), blocking the entry of pyruvate into the mitochondria inhibits formation of acetyl CoA and slows the Krebs’ cycle[30]. In contrast to the normal RV, which can vary its substrate utilization from fatty acids to glucose as needed, RVH is associated with a persistent reliance on glucose metabolism[27]. Dichloroacetate, a prototypic inhibitor of PDK activates PDH, thereby increasing acetyl coenzyme A and enhancing Krebs’ cycle[31]. The 14C-glucose technique[25] showed that dichloroacetate rapidly increases glucose oxidation and concordantly enhances RV function (Fig. 2), which in light of the constant afterload in this model is consistent with an increase in contractility. Dichloroacetate’s ability to increase O2 consumption (by enhancing glucose oxidation) was evident in both the monocrotaline and PAB models (Fig. 3A and 8B). The relatively greater increase in glucose oxidation relative to glycolysis caused by dichloroacetate reflects better coupling and would be predicted to reduce lactate and proton production. This has the potential to increase cardiac efficiency, since less energy is used to correct this ionic imbalance.
The beneficial effects of chronic dichloroacetate likely relate both to the enhanced myocardial energetics that occurs when oxidative metabolism is enhanced and the restoration of Kv channel expression. In the working heart model, dichloroacetate rapidly improves RV function (Fig. 2A). This improvement is too rapid to attribute to transcriptional restoration of Kv expression. We also show that dichloroacetate does not acutely shorten MAPD-Supplemental Figure 2). Moreover, expression of the Glut1 transporter was also normalized by dichloroacetate in both models (Fig. 4 A, B&C and Fig. 8A). We believe that the upregulated Glut1 and impaired oxygen consumption in RVH reflect the need to increase glucose import in RVH to compensate for the lower yield of ATP in glycolysis vs oxidative glucose metabolism.
Published evidence for impaired metabolism in RVH is limited. However, a proteomic study of monocrotaline-induced RVH found a pattern of enzyme expression consistent with impaired oxidative metabolism, including down-regulation of PDH (subunit beta E1), isocitrate dehydrogenase, succinyl coenzyme A ligase, NADH dehydrogenase, ubiquinol-cytochrome C reductase, and propionyl coenzyme A carboxylase[32]. We noted a similar reversible mitochondrial metabolic abnormality in the pulmonary vasculature in PAH and in human cancers[1, 19]. In both cases these abnormalities are initiated and maintained, in part, by pathological PDK activation and in both cases several weeks of dichloroacetate therapy is beneficial, regressing vascular obstruction in PAH[1] and stopping tumor growth in a xenotransplantation model of human lung cancer[19]. Similar benefits of PDK inhibition have been reported in experimental LVH[21, 33] and myocardial infarction[22], conditions associated with enhanced glycolysis [21, 33].
Dichloroacetate and electrical remodeling in RVH
Cellular electrophysiological studies have shown that repolarization abnormalities noted on EKG in animals and humans with ventricular hypertrophy are caused by depression of K+ currents[34]. It is an underappreciated fact that patients with RVH have mild prolongation of the QTc interval, particularly on right-sided leads[8]. A decrease in the IKr in RVH appears to explain the prolongation of MAPD[28]. In present study, multiple Kv channels including Kv1.2, Kv1.5 and Kv4.2 were downregulated in RVH and this was accompanied with the prolongation of MAPD and the QTc interval. Inhibition of repolarizing K+ currents and MAPD prolongation likely adds to the negative inotropic effects of glycolytic metabolism and further reduces RV contractile function [10] (Fig. 8D). Conversely, dichloroacetate’s beneficial electrical effects are, at least in part, due to increased expression of several repolarizing Kv channels, including Kv4.2 and Kv 1.5 (Fig. 6 B, C, E&F). Dichloroacetate’s effect in shortening MAPD20 may relate to its ability to increase expression of both Kv4.2 and Kv1.5 which have the properties of fast activation[6]. Acutely, application of dichloroacetate does not shorten MAPD, consistent with the dichloroacetate’s effects on MAPD relating to re-expression of Kv channels, rather than enhanced channel activation (Supplemental Fig. 2). This suggests that the changes in mitochondrial metabolism induced by PDK contribute significantly to the electrical remodeling in RVH induced by PAH. Consistent with our findings, in a patch clamp of surviving cardiomyocytes from infarcted rat heart, dichloroacetate (1.5 mM) increases the depressed Ito current density; whereas 3-bromopyruvate, a PDH complex inhibitor, reduces the current density[22]. Together with our observations, the literature[22] supports the hypothesis that the downregulation of Kv channels that underlies reduced K+ currents in RVH reflects transcriptional effects induced by PDK-mediated metabolic changes.
Limitations
We realize that our data on the metabolic shift and electrical remodeling in RVH is only one step towards a better understanding of the pathophysiology in RVH and RV failure. The initiating stimulus for PDK activation is uncertain. However, since these changes occur only when the experimental RVH is severe we speculate that it reflects a supply-demand mismatch in coronary perfusion of the RV and its increased metabolic demands. Consistent with this, several papers suggest that the hypertrophied RV may be ischemic[2, 35]. Future studies are required to determine the mechanism by which inhibition of PDH downregulates expression of the repolarizing Kv channels. Additional studies are also planed to assess other metabolic therapies in RVH, including agents that alter fatty acid oxidation, such as trimetazidine.
Although dichloroacetate has been safely used in humans to treat lactic acidosis related to mitochondrial diseases [36], the long-term safety of dichloroacetate in patients with RVH remains to be determined. Moreover, the safety of drugs that reduce hypertrophy, traditionally thought to be a beneficial compensatory mechanism in conditions of increased afterload, remains to be established. In the case of dichloroacetate we believe the fact that reduction in RVH relates to positive changes in vascular remodeling and myocardial energetics favors the interpretation that regression of RVH reflects disease regression and is thus “beneficial”.
While we found that alpha actin protein did not increase in RVH, Bakerman et al. noted an increase in alpha-skeletal actin mRNA in the RV of calves exposed to acute hypoxia[37]. While an RVH-associated increase in the reporter protein is a theoretical concern, and could have complicated interpretation of the GLUT immunoblot, no increase in actin was observed. The differences between Bakerman’s study and ours include the species and model of RVH used and the fact we measured protein while they measured mRNA. Changes in mRNA do not always translate into changes in protein. Finally Zhang et al., also using a rodent model, found no change in actin expression in a monocrotaline rodent model[7].
Conclusion
Electrical remodeling and RV dysfunction in RVH due to PAH and PAB relate to a glycolytic shift mediated, in large part, by PDK activation and inhibition of the PDH complex. Dichloroacetate, reverses the electrical remodeling and enhances RV function by enhancing glucose oxidation (Fig. 8D). The relevance of these findings is enhanced by the fact that dichloroacetate has been safely and chronically administered in humans[36], suggesting a trial in RVH associated with PAH or pulmonic stenosis could be safely conducted.
Supplementary Material
ACKNOWLEDGEMENT
Dr. Archer is supported by The Harold Hines Jr. Chair in Department of Medicine in University of Chicago and NIH-RO1-HL071115. We appreciate the assistance of Dr. William Green and his PhD student Ning Zheng in the immunoblotting studies and Judy U. Earley in the preparation of H&E staining.
Footnotes
DISCLOSURES
The authors have no conflicts to disclose.
REFERENCES
- 1.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thebaud B, Haromy A, Harry G, Moudgil R, McMurtry MS, Weir EK, Archer SL. An abnormal mitochondrial-hypoxia inducible factor-1alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation. 2006;113:2630–2641. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- 2.van Wolferen SA, Marcus JT, Boonstra A, Marques KM, Bronzwaer JG, Spreeuwenberg MD, Postmus PE, Vonk-Noordegraaf A. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J. 2007;28:1250–1257. doi: 10.1093/eurheartj/ehl477. [DOI] [PubMed] [Google Scholar]
- 3.Hessel MH, Steendijk P, den Adel B, Schutte CI, van der Laarse A. Characterization of right ventricular function after monocrotaline-induced pulmonary hypertension in the intact rat. Am J Physiol Heart Circ Physiol. 2006;291:H2424–H2430. doi: 10.1152/ajpheart.00369.2006. [DOI] [PubMed] [Google Scholar]
- 4.Lamberts RR, Caldenhoven E, Lansink M, Witte G, Vaessen RJ, St Cyr JA, Stienen GJ. Preservation of diastolic function in monocrotaline-induced right ventricular hypertrophy in rats. Am J Physiol Heart Circ Physiol. 2007;293:H1869–H1876. doi: 10.1152/ajpheart.00294.2007. [DOI] [PubMed] [Google Scholar]
- 5.Lee JK, Kodama I, Honjo H, Anno T, Kamiya K, Toyama J. Stage-dependent changes in membrane currents in rats with monocrotaline-induced right ventricular hypertrophy. Am J Physiol. 1997;272:H2833–H2842. doi: 10.1152/ajpheart.1997.272.6.H2833. [DOI] [PubMed] [Google Scholar]
- 6.Lee JK, Nishiyama A, Kambe F, Seo H, Takeuchi S, Kamiya K, Kodama I, Toyama J. Downregulation of voltage-gated K(+) channels in rat heart with right ventricular hypertrophy. Am J Physiol. 1999;277:H1725–H1731. doi: 10.1152/ajpheart.1999.277.5.H1725. [DOI] [PubMed] [Google Scholar]
- 7.Zhang TT, Cui B, Dai DZ. Downregulation of Kv4.2 and Kv4.3 channel gene expression in right ventricular hypertrophy induced by monocrotaline in rat. Acta Pharmacol Sin. 2004;25:226–230. [PubMed] [Google Scholar]
- 8.Hlaing T, Guo D, Zhao X, DiMino T, Greenspon L, Kowey PR, Yan GX. The QT and Tp-e intervals in left and right chest leads: comparison between patients with systemic and pulmonary hypertension. J Electrocardiol. 2005;38:154–158. doi: 10.1016/j.jelectrocard.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 9.De Ambroggi L, Francia P, De Ambroggi G. Repolarization abnormalities and arrhythmogenesis in hypertrophic myocardium. Anadolu Kardiyol Derg. 2007;7 Suppl 1:71–72. [PubMed] [Google Scholar]
- 10.Kaprielian R, Wickenden AD, Kassiri Z, Parker TG, Liu PP, Backx PH. Relationship between K+ channel down-regulation and [Ca2+]i in rat ventricular myocytes following myocardial infarction. J Physiol. 1999;517(Pt 1):229–245. doi: 10.1111/j.1469-7793.1999.0229z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wood EH. Action potential control of cardiac contractility. Ann Biomed Eng. 2000;28:860–870. doi: 10.1114/1.1313772. [DOI] [PubMed] [Google Scholar]
- 12.Harris DM, Mills GD, Chen X, Kubo H, Berretta RM, Votaw VS, Santana LF, Houser SR. Alterations in early action potential repolarization causes localized failure of sarcoplasmic reticulum Ca2+ release. Circ Res. 2005;96:543–550. doi: 10.1161/01.RES.0000158966.58380.37. [DOI] [PubMed] [Google Scholar]
- 13.Oikawa M, Kagaya Y, Otani H, Sakuma M, Demachi J, Suzuki J, Takahashi T, Nawata J, Ido T, Watanabe J, Shirato K. Increased [18F]fluorodeoxyglucose accumulation in right ventricular free wall in patients with pulmonary hypertension and the effect of epoprostenol. J Am Coll Cardiol. 2005;45:1849–1855. doi: 10.1016/j.jacc.2005.02.065. [DOI] [PubMed] [Google Scholar]
- 14.Basu S, Alzeair S, Li G, Dadparvar S, Alavi A. Etiopathologies associated with intercostal muscle hypermetabolism and prominent right ventricle visualization on 2-deoxy-2[F-18]fluoro-D-glucose-positron emission tomography: significance of an incidental finding and in the setting of a known pulmonary disease. Mol Imaging Biol. 2007;9:333–339. doi: 10.1007/s11307-007-0102-7. [DOI] [PubMed] [Google Scholar]
- 15.Hill NS, Jederlinic P, Gagnon J. Supplemental oxygen reduces right ventricular hypertrophy in monocrotaline-injected rats. J Appl Physiol. 1989;66:1642–1648. doi: 10.1152/jappl.1989.66.4.1642. [DOI] [PubMed] [Google Scholar]
- 16.Xu W, Koeck T, Lara AR, Neumann D, DiFilippo FP, Koo M, Janocha AJ, Masri FA, Arroliga AC, Jennings C, Dweik RA, Tuder RM, Stuehr DJ, Erzurum SC. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc Natl Acad Sci U S A. 2007;104:1342–1347. doi: 10.1073/pnas.0605080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knoechel TR, Tucker AD, Robinson CM, Phillips C, Taylor W, Bungay PJ, Kasten SA, Roche TE, Brown DG. Regulatory roles of the N-terminal domain based on crystal structures of human pyruvate dehydrogenase kinase 2 containing physiological and synthetic ligands. Biochemistry. 2006;45:402–415. doi: 10.1021/bi051402s. [DOI] [PubMed] [Google Scholar]
- 18.Michelakis ED, McMurtry MS, Wu XC, Dyck JR, Moudgil R, Hopkins TA, Lopaschuk GD, Puttagunta L, Waite R, Archer SL. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation. 2002;105:244–250. doi: 10.1161/hc0202.101974. [DOI] [PubMed] [Google Scholar]
- 19.Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Harry G, Hashimoto K, Porter CJ, Andrade MA, Thebaud B, Michelakis ED. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 20.Allard MF, Schonekess BO, Henning SL, English DR, Lopaschuk GD. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol. 1994;267:H742–H750. doi: 10.1152/ajpheart.1994.267.2.H742. [DOI] [PubMed] [Google Scholar]
- 21.Wambolt RB, Lopaschuk GD, Brownsey RW, Allard MF. Dichloroacetate improves postischemic function of hypertrophied rat hearts. J Am Coll Cardiol. 2000;36:1378–1385. doi: 10.1016/s0735-1097(00)00856-1. [DOI] [PubMed] [Google Scholar]
- 22.Rozanski GJ, Xu Z, Zhang K, Patel KP. Altered K+ current of ventricular myocytes in rats with chronic myocardial infarction. Am J Physiol. 1998;274:H259–H265. doi: 10.1152/ajpheart.1998.274.1.H259. [DOI] [PubMed] [Google Scholar]
- 23.Knollmann BC, Katchman AN, Franz MR. Monophasic action potential recordings from intact mouse heart: validation, regional heterogeneity, and relation to refractoriness. J Cardiovasc Electrophysiol. 2001;12:1286–1294. doi: 10.1046/j.1540-8167.2001.01286.x. [DOI] [PubMed] [Google Scholar]
- 24.Liu B, Clanachan AS, Schulz R, Lopaschuk GD. Cardiac efficiency is improved after ischemia by altering both the source and fate of protons. Circ Res. 1996;79:940–948. doi: 10.1161/01.res.79.5.940. [DOI] [PubMed] [Google Scholar]
- 25.Barr RL, Lopaschuk GD. Methodology for measuring in vitro/ex vivo cardiac energy metabolism. J Pharmacol Toxicol Methods. 2000;43:141–152. doi: 10.1016/s1056-8719(00)00096-4. [DOI] [PubMed] [Google Scholar]
- 26.Abel ED. Glucose transport in the heart. Front Biosci. 2004;9:201–215. doi: 10.2741/1216. [DOI] [PubMed] [Google Scholar]
- 27.Sharma S, Taegtmeyer H, Adrogue J, Razeghi P, Sen S, Ngumbela K, Essop MF. Dynamic changes of gene expression in hypoxia-induced right ventricular hypertrophy. Am J Physiol Heart Circ Physiol. 2004;286:H1185–H1192. doi: 10.1152/ajpheart.00916.2003. [DOI] [PubMed] [Google Scholar]
- 28.Kleiman RB, Houser SR. Outward currents in normal and hypertrophied feline ventricular myocytes. Am J Physiol. 1989;256:H1450–H1461. doi: 10.1152/ajpheart.1989.256.5.H1450. [DOI] [PubMed] [Google Scholar]
- 29.Nagendran J, Gurtu V, Fu DZ, Dyck JR, Haromy A, Ross DB, Rebeyka IM, Michelakis ED. A dynamic and chamber-specific mitochondrial remodeling in right ventricular hypertrophy can be therapeutically targeted. J Thorac Cardiovasc Surg. 2008;136:168–178. doi: 10.1016/j.jtcvs.2008.01.040. 178 e161-163. [DOI] [PubMed] [Google Scholar]
- 30.Sugden MC, Langdown ML, Harris RA, Holness MJ. Expression and regulation of pyruvate dehydrogenase kinase isoforms in the developing rat heart and in adulthood: role of thyroid hormone status and lipid supply. Biochem J. 2000;352(Pt 3):731–738. [PMC free article] [PubMed] [Google Scholar]
- 31.Clarke B, Wyatt KM, McCormack JG. Ranolazine increases active pyruvate dehydrogenase in perfused normoxic rat hearts: evidence for an indirect mechanism. J Mol Cell Cardiol. 1996;28:341–350. doi: 10.1006/jmcc.1996.0032. [DOI] [PubMed] [Google Scholar]
- 32.Schott P, Singer SS, Kogler H, Neddermeier D, Leineweber K, Brodde OE, Regitz-Zagrosek V, Schmidt B, Dihazi H, Hasenfuss G. Pressure overload and neurohumoral activation differentially affect the myocardial proteome. Proteomics. 2005;5:1372–1381. doi: 10.1002/pmic.200401005. [DOI] [PubMed] [Google Scholar]
- 33.Lydell CP, Chan A, Wambolt RB, Sambandam N, Parsons H, Bondy GP, Rodrigues B, Popov KM, Harris RA, Brownsey RW, Allard MF. Pyruvate dehydrogenase and the regulation of glucose oxidation in hypertrophied rat hearts. Cardiovasc Res. 2002;53:841–851. doi: 10.1016/s0008-6363(01)00560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swynghedauw B, Baillard C, Milliez P. The long QT interval is not only inherited but is also linked to cardiac hypertrophy. J Mol Med. 2003;81:336–345. doi: 10.1007/s00109-003-0437-8. [DOI] [PubMed] [Google Scholar]
- 35.Saito D, Shiraki T, Inoue K, Kajiyama A, Takemoto S, Hori S, Takamura T, Kono H. Reduced vasodilator response of the right coronary artery to myocardial ischemia in the hypertrophied right ventricle. Jpn Circ J. 1996;60:247–253. doi: 10.1253/jcj.60.247. [DOI] [PubMed] [Google Scholar]
- 36.Stacpoole PW, Kerr DS, Barnes C, Bunch ST, Carney PR, Fennell EM, Felitsyn NM, Gilmore RL, Greer M, Henderson GN, Hutson AD, Neiberger RE, O'Brien RG, Perkins LA, Quisling RG, Shroads AL, Shuster JJ, Silverstein JH, Theriaque DW, Valenstein E. Controlled clinical trial of dichloroacetate for treatment of congenital lactic acidosis in children. Pediatrics. 2006;117:1519–1531. doi: 10.1542/peds.2005-1226. [DOI] [PubMed] [Google Scholar]
- 37.Bakerman PR, Stenmark KR, Fisher JH. Alpha-skeletal actin messenger RNA increases in acute right ventricular hypertrophy. Am J Physiol. 1990;258:L173–L178. doi: 10.1152/ajplung.1990.258.4.L173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.