Abstract
Previous studies in our laboratory and others have demonstrated in humans and other mammals two isozymes of arginase (AI and AII) that differ both electrophoretically and antigenically. AI, a cytosolic protein found predominantly in liver and red blood cells, is believed to be chiefly responsible for ureagenesis and is the one missing in hyperargininemic patients. Much less is known about AII because it is present in far smaller amounts and localized in less accessible deep tissues, primarily kidney. We now report the application of enzymatic and immunologic methods to assess the independent expression and regulation of these two gene products in normal tissue extracts, two cultured cell lines, and multiple organ samples from a hyperargininemic patient who came to autopsy after an unusually severe clinical course characterized by rapidly progressive hepatic cirrhosis. AI was totally absent (less than 0.1%) in the patient's tissues, whereas marked enhancement of AII activity (four times normal) was seen in the kidney by immunoprecipitation and biochemical inhibition studies. Immunoprecipitation-competition and Western blot analysis failed to reveal presence of even an enzymatically inactive cross-reacting AI protein, whereas Southern blot analysis showed no evidence of a substantial deletion in the AI gene. Induction studies in cell lines that similarly express only the AII isozyme indicated that its activity could be enhanced severalfold by exposure to elevated arginine levels. Our findings suggest that the same induction mechanism may well be operative in hyperargininemic patients, and that the heightened AII activity may be responsible for the persistent ureagenesis seen in this disorder. These data lend further support to the existence of two separate arginase gene loci in humans, and raise possibilities for novel therapeutic approaches based on their independent manipulation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bascur L., Cabello J., Véliz M., González A. Molecular forms of human-liver arginase. Biochim Biophys Acta. 1966 Oct 17;128(1):149–154. doi: 10.1016/0926-6593(66)90151-2. [DOI] [PubMed] [Google Scholar]
- Bernar J., Hanson R. A., Kern R., Phoenix B., Shaw K. N., Cederbaum S. D. Arginase deficiency in a 12-year-old boy with mild impairment of intellectual function. J Pediatr. 1986 Mar;108(3):432–435. doi: 10.1016/s0022-3476(86)80891-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CABELLO J., BASILIO C., PRAJOUX V. Kinetic properties of erythrocyte- and liver arginase. Biochim Biophys Acta. 1961 Mar 18;48:148–152. doi: 10.1016/0006-3002(61)90525-x. [DOI] [PubMed] [Google Scholar]
- Cabello J., Prajoux V., Plaza M. Immunodiffusion studies on human liver and erythrocyte arginases. Biochim Biophys Acta. 1965 Sep 20;105(3):583–593. doi: 10.1016/s0926-6593(65)80241-7. [DOI] [PubMed] [Google Scholar]
- Carvajal N., Cederbaum S. D. Kinetics of inhibition of rat liver and kidney arginases by proline and branched-chain amino acids. Biochim Biophys Acta. 1986 Mar 28;870(2):181–184. doi: 10.1016/0167-4838(86)90219-0. [DOI] [PubMed] [Google Scholar]
- Cederbaum S. D., Shaw K. N., Spector E. B., Verity M. A., Snodgrass P. J., Sugarman G. I. Hyperargininemia with arginase deficiency. Pediatr Res. 1979 Jul;13(7):827–833. doi: 10.1203/00006450-197907000-00007. [DOI] [PubMed] [Google Scholar]
- Cederbaum S. D., Shaw K. N., Valente M. Hyperargininemia. J Pediatr. 1977 Apr;90(4):569–573. doi: 10.1016/s0022-3476(77)80368-5. [DOI] [PubMed] [Google Scholar]
- Chu S. W., Nesheim M. C. The relationship of plasma arginine and kidney arginase activity to arginine degradation in chickens. J Nutr. 1979 Oct;109(10):1752–1758. doi: 10.1093/jn/109.10.1752. [DOI] [PubMed] [Google Scholar]
- Dizikes G. J., Grody W. W., Kern R. M., Cederbaum S. D. Isolation of human liver arginase cDNA and demonstration of nonhomology between the two human arginase genes. Biochem Biophys Res Commun. 1986 Nov 26;141(1):53–59. doi: 10.1016/s0006-291x(86)80333-3. [DOI] [PubMed] [Google Scholar]
- Dizikes G. J., Spector E. B., Cederbaum S. D. Cloning of rat liver arginase cDNA and elucidation of regulation of arginase gene expression in H4 rat hepatoma cells. Somat Cell Mol Genet. 1986 Jul;12(4):375–384. doi: 10.1007/BF01570732. [DOI] [PubMed] [Google Scholar]
- Gasiorowska I., Porembska Z., Jachimowicz J., Mochnacka I. Isoenzymes of arginase in rat tissues. Acta Biochim Pol. 1970;17(1):19–30. [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Haggerty D. F., Spector E. B., Lynch M., Kern R., Frank L. B., Cederbaum S. D. Regulation of glucocorticoids of arginase and argininosuccinate synthetase in cultured rat hepatoma cells. J Biol Chem. 1982 Mar 10;257(5):2246–2253. [PubMed] [Google Scholar]
- Herrmann B. G., Frischauf A. M. Isolation of genomic DNA. Methods Enzymol. 1987;152:180–183. doi: 10.1016/0076-6879(87)52018-3. [DOI] [PubMed] [Google Scholar]
- Herzfeld A., Raper S. M. The heterogeneity of arginases in rat tissues. Biochem J. 1976 Feb 1;153(2):469–478. doi: 10.1042/bj1530469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordá A., Portolés M., Rubio V., Capdevila A., Vilas J., García-Piño J. Liver fibrosis in arginase deficiency. Arch Pathol Lab Med. 1987 Aug;111(8):691–692. [PubMed] [Google Scholar]
- Kawamoto S., Amaya Y., Oda T., Kuzumi T., Saheki T., Kimura S., Mori M. Cloning and expression in Escherichia coli of cDNA for arginase of rat liver. Biochem Biophys Res Commun. 1986 May 14;136(3):955–961. doi: 10.1016/0006-291x(86)90425-0. [DOI] [PubMed] [Google Scholar]
- Kaysen G. A., Strecker H. J. Purification and properties of arginase of rat kidney. Biochem J. 1973 Aug;133(4):779–788. doi: 10.1042/bj1330779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel L. M., Smith K. D., Boyer S. H., Borgaonkar D. S., Wachtel S. S., Miller O. J., Breg W. R., Jones H. W., Jr, Rary J. M. Analysis of human Y-chromosome-specific reiterated DNA in chromosome variants. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1245–1249. doi: 10.1073/pnas.74.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezl V. A., Knox W. E. Metabolism of arginine in lactating rat mammary gland. Biochem J. 1977 Jul 15;166(1):105–113. doi: 10.1042/bj1660105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels V. V., Beaudet A. L. Arginase deficiency in multiple tissues in argininemia. Clin Genet. 1978 Jan;13(1):61–67. doi: 10.1111/j.1399-0004.1978.tb04128.x. [DOI] [PubMed] [Google Scholar]
- SCHIMKE R. T. ENZYMES OF ARGININE METABOLISM IN MAMMALIAN CELL CULTURE. I. REPRESSION OF ARGININOSUCCINATE SYNTHETASE AND ARGININOSUCCINASE. J Biol Chem. 1964 Jan;239:136–145. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sparkes R. S., Dizikes G. J., Klisak I., Grody W. W., Mohandas T., Heinzmann C., Zollman S., Lusis A. J., Cederbaum S. D. The gene for human liver arginase (ARG1) is assigned to chromosome band 6q23. Am J Hum Genet. 1986 Aug;39(2):186–193. [PMC free article] [PubMed] [Google Scholar]
- Spector E. B., Kern R. M., Haggerty D. F., Cederbaum S. D. Differential expression of multiple forms of arginase in cultured cells. Mol Cell Biochem. 1985 Feb;66(1):45–53. doi: 10.1007/BF00231822. [DOI] [PubMed] [Google Scholar]
- Spector E. B., Kiernan M., Bernard B., Cederbaum S. D. Properties of fetal and adult red blood cell arginase: a possible prenatal diagnostic test for arginase deficiency. Am J Hum Genet. 1980 Jan;32(1):79–87. [PMC free article] [PubMed] [Google Scholar]
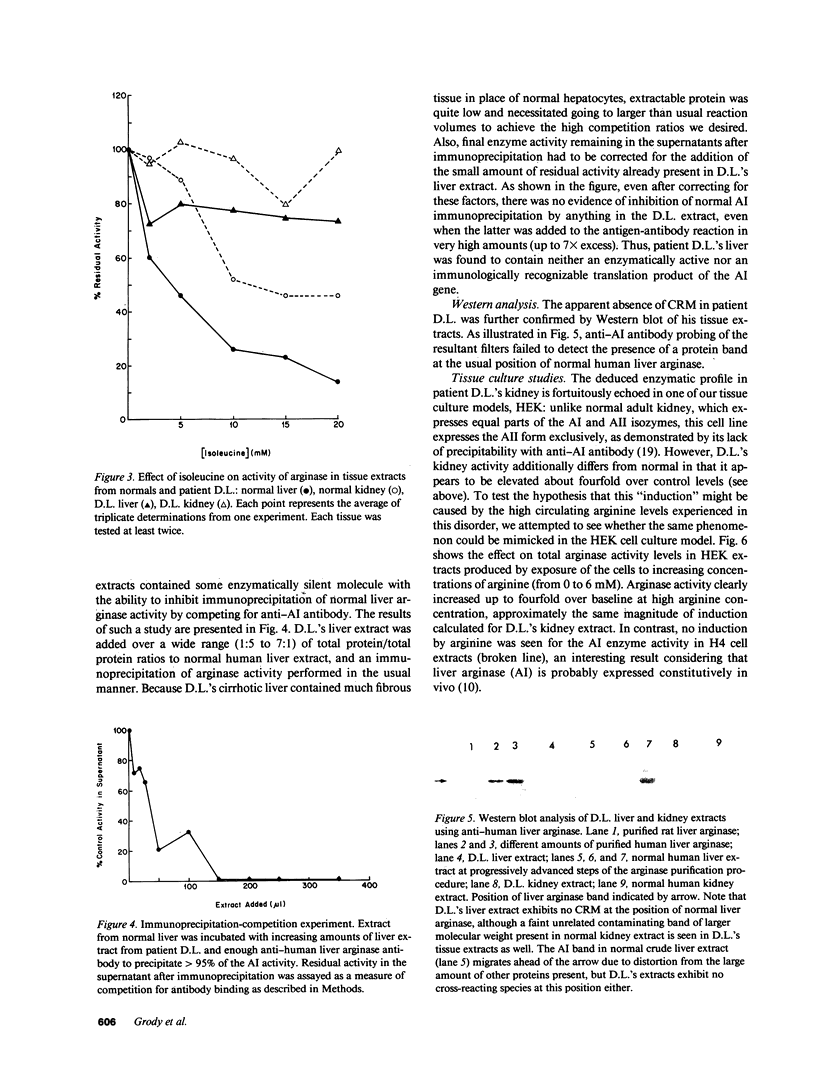
- Spector E. B., Rice S. C., Cederbaum S. D. Immunologic studies of arginase in tissues of normal human adult and arginase-deficient patients. Pediatr Res. 1983 Dec;17(12):941–944. doi: 10.1203/00006450-198312000-00003. [DOI] [PubMed] [Google Scholar]
- Spector E. B., Rice S. C., Moedjono S., Bernard B., Cederbaum S. D. Biochemical properties of arginase in human adult and fetal tissues. Biochem Med. 1982 Oct;28(2):165–175. doi: 10.1016/0006-2944(82)90067-9. [DOI] [PubMed] [Google Scholar]
- Symington J., Green M., Brackmann K. Immunoautoradiographic detection of proteins after electrophoretic transfer from gels to diazo-paper: analysis of adenovirus encoded proteins. Proc Natl Acad Sci U S A. 1981 Jan;78(1):177–181. doi: 10.1073/pnas.78.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner M. S., Waithe W. I., Hirschhorn K. L-asparaginase and blastogenesis. Lancet. 1969 Oct 4;2(7623):748–748. doi: 10.1016/s0140-6736(69)90464-4. [DOI] [PubMed] [Google Scholar]
- Zimmermann A., Bachmann C., Baumgartner R. Severe liver fibrosis in argininosuccinic aciduria. Arch Pathol Lab Med. 1986 Feb;110(2):136–140. [PubMed] [Google Scholar]