Abstract
Interleukin-1β (IL-1β) is a pleiotropic cytokine promoting inflammation, angiogenesis, and tissue remodeling as well as regulation of immune responses. Although IL-1β contributes to growth and metastatic spread in experimental and human cancers, the molecular mechanisms regulating the conversion of the inactive IL-1β precursor to a secreted and active cytokine remains unclear. Here we demonstrate that NALP3 inflammasome is constitutively assembled and activated with cleavage of caspase-1 in human melanoma cells. Late stage human melanoma cells spontaneously secrete active IL-1β via constitutive activation of the NALP3 inflammasome and IL-1 receptor signaling, exhibiting a feature of autoinflammatory diseases. Unlike human blood monocytes, these melanoma cells require no exogenous stimulation. In contrast, NALP3 functionality in intermediate stage melanoma cells requires activation of the IL-1 receptor to secrete active IL-1β; cells from an early stage of melanoma require stimulation of the IL-1 receptor plus the co-stimulant muramyl dipeptide. The spontaneous secretion of IL-1β from melanoma cells was reduced by inhibition of caspase-1 or the use of small interfering RNA directed against ASC. Supernatants from melanoma cell cultures enhanced macrophage chemotaxis and promoted in vitro angiogenesis, both prevented by pretreating melanoma cells with inhibitors of caspases-1 and -5 or IL-1 receptor blockade. These findings implicate IL-1-mediated autoinflammation as contributing to the development and progression of human melanoma and suggest that inhibiting the inflammasome pathway or reducing IL-1 activity can be a therapeutic option for melanoma patients.
Keywords: Cancer, Cytokines, Immunology, Caspase, Inflammation, Autoinflammation, Caspase-1, Inflammasome, Interleukin
Introduction
In humans and mice, tumors secrete pro-inflammatory cytokines, chemokines, and other soluble factors in the tumor microenvironment, thereby promoting tumor development and progression (1–3). Interleukin-1β (IL-1β)3 is a pleiotropic pro-inflammatory cytokine involved in cell growth, differentiation, tissue repair, and regulation of immune response (4). IL-1β is often detected in human cancer tissues including breast cancer, pancreatic cancer, glioblastoma, and melanoma (5–13), where the expression levels of the IL-1β protein or gene are associated with the invasiveness and metastasis of cancers. Experiments using exogenously administered IL-1 or IL-1 knock-out mice suggest that stromal-derived IL-1 plays an important role in the progression of murine tumors (14–19), whereas recent reports have shown that tumor-derived IL-1β is associated with tumor growth, immunosuppression, and chemoresistance (7, 8, 13, 15). Considering the large portfolio of genes induced by IL-1 (4), it is thus critical to elucidate the biological mechanisms responsible for the dysregulated secretion of IL-1β in human cancer cells.
IL-1β is first synthesized as biologically inactive precursor (pro-IL-1β) in response to Toll-like receptor (TLR) agonists in macrophages (4). Pro-IL-1β is then cleaved by caspase-1 to biologically active mature IL-1β, resulting in its release into the extracellular space, where it initiates the inflammatory response (4, 20). A pioneering study to investigate the molecular mechanisms controlling caspase-1 activity led to the identification and characterization of the “inflammasome,” an elaborate multi-protein complex whose assembly and activation is responsible for the recruitment and activation of caspases-1 and -5 (21). Each inflammasome includes members of the nucleotide oligomerization domain-like receptor (NLR) family of proteins. Diverse pathogen-associated molecular patterns and nonmicrobial danger-associated molecular patterns are sensed intracellularly by NLRs, resulting in oligomerization of NLRs. Following oligomerization, NLRs interacts with ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain (CARD)), a central adaptor protein of inflammasome, through homotypic interactions of pyrin domain. ASC then interacts with pro-caspase-1 through its homotypic interactions of CARD domain, yielding cleavage and activation of caspase-1, which cleaves pro-IL-1β to the active IL-1β (22–25). NALP (NACHT, LRR, and pyrin domain-containing protein) 1 inflammasome and NALP2/3 inflammasome are two of the best characterized human inflammasomes (22–25). Constitutive activation of NALP3 inflammasome caused by the single amino acid mutations in the NALP3 gene leads to several autoinflammatory diseases characterized by sustained local and systemic inflammation mediated by IL-1β (26–29).
In the present study, we provide evidence that constitutively activated inflammasome mediates the conversion of the cytoplasmic (inactive) IL-1β precursor to a secreted and active form of IL-1β in human melanoma cells. Furthermore, we demonstrate that human melanoma cells from the late stage of the disease spontaneously secrete biologically active IL-1β in the absence of exogenous stimuli because of constitutive activation of the inflammasome and IL-1 receptor (IL-1R) signaling. This feature falls into the category of autoinflammatory diseases, which may be unique to late stage melanoma.
EXPERIMENTAL PROCEDURES
Chemicals and Reagents
Caspase inhibitors (Z-VAD-FMK, Z-YVAD-FMK, and Z-WEHD-FMK) were obtained from Alexis Biochemicals (San Diego, CA), and IL-1Ra (anakinra, Kineret®) was purchased from Amgen, Inc. (Thousand Oaks, CA). Lipopolysaccharide (LPS), phorbol 12-myristate13-acetate (PMA), and muramyl dipeptide were purchased from Sigma-Aldrich. Recombinant human IL-1α was from eBioscience (San Diego, CA). Protein G-Sepharose beads were purchased from Invitrogen.
Antibodies
Anti-caspase-5 antibody (ab17825, rabbit polyclonal) was obtained from Abcam, Inc. (Cambridge, MA). Anti-human IL-1β antibody (affinity-purified goat polyclonal) and anti-caspase-1 p20 antibody (2225, rabbit polyclonal) were purchased from R & D Systems (Minneapolis, MN) and Cell Signaling Technology, Inc. (Danvers, MA), respectively. Anti-ASC antibody (rabbit polyclonal), anti-NALP1 antibody (mouse monoclonal) and anti-NALP3 antibody (mouse monoclonal) were from Alexis Biochemicals. Horseradish peroxidase-conjugated secondary antibody (anti-mouse IgG) was purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA), and horseradish peroxidase-conjugated secondary antibodies (anti-rabbit IgG and anti-goat IgG) were from Sigma-Aldrich.
Cell Culture
Human melanoma cell lines were obtained from the American Type Culture Collection (Manassas, VA). They were established from different stages of disease progression in the laboratory of Dr. Meenhard Herlyn (Wistar Institute, Philadelphia, PA) with the exception of A375. WM35 and WM1552C are from “early stage melanoma” such as radial growth phase (RGP). Patients were cured by surgical removal of these primary lesions. Others are metastatically competent melanomas, either from “intermediate stage melanoma” such as vertical growth phase (VGP) primary tumors whose patients developed metastasis later (WM75, WM115, WM278, and WM793B) or from “late stage melanoma” such as distant metastasis (A375, HS294T, and 1205Lu). Melanoma cells were maintained in RPMI 1640 (Mediatech, Inc., Manassas, VA) (except HS294T) or Dulbecco's modified Eagle's medium (Mediatech, Inc.) (for HS294T) supplemented with 10% fetal bovine serum (Mediatech, Inc.), 100 units/ml penicillin, 0.1 mg/ml streptomycin, and 2 mm l-glutamine.
The THP-1 cell line (derived from an acute monocytic leukemia patient) was differentiated with 50 nm (31 ng/ml) PMA for 24 h before each experiment. Human umbilical vein endothelial cells (HUVECs) (Astarte Biologics, Redmond, WA) were maintained in growth medium (catalog number 2015; Astarte Biologics).
Clinical Melanoma Specimens
Human melanoma tissues were obtained from surgical specimens with patient consent under institutional review board-approved protocols, adhering to Health Insurance Portability and Accountability Act regulations. The tumors were processed for histological and biological analyses.
Immunohistochemistry
Immunohistochemical studies were performed on formalin-fixed paraffin embedded tissues using a goat polyclonal antibody against human IL-1β (R & D Systems). Briefly, 4-μm-thick paraffin sections were deparaffinized and hydrated. Heat-induced antigen retrieval was performed in 0.01 m citrate buffer (Antigen retrieval citra solution; BioGenex Laboratories, San Ramon, CA). The sections were treated with 0.3% hydrogen peroxide to block endogenous peroxidase activity and incubated with 10 μg/ml of primary antibody for overnight at 4 °C, followed by biotinylated donkey anti-goat IgG for 30 min and ABComplex/horseradish peroxidase avidin-biotinylated peroxidase complex (Vector Laboratories) for 30 min. The immunoreactants were visualized with diaminobenzidine substrate. Hematoxylin (Sigma-Aldrich) was used for counter staining.
Clinical Sample Preparation
Human tumors were minced into small pieces (∼2 mm3). Single cell suspensions were generated by enzymatic digestion with 2 mg/ml collagenase (Sigma-Aldrich) and 2 mg/ml hyaluronidase (Sigma-Aldrich) for 2 h at 37 °C with intermittent vortex, followed by sequentially passing through 70- and 40-μm filters. Red blood cells were lysed using 1× red blood cell lysis buffer (eBioscience). Dead cells were removed with a dead cell removal kit (Miltenyi Biotec, Auburn, CA). The cells were washed and subjected to biological analyses including ELISA and Western blotting.
Cytokine Production and Cytokine Determinations
The cells were cultured in OptiMEM (Invitrogen) at 80% confluence. After 24 h, the supernatants (secreted IL-1β) were collected and centrifuged at 210 × g for 5 min. Intracellular IL-1β was assessed by lysing cells with 0.5% Triton X-100 in phosphate-buffered saline. The lysate was then subjected to a freeze-thaw cycle followed by centrifugation at 15,000 × g for 10 min at 4 °C.
Supernatants and cell lysates were analyzed by ELISA for IL-1β, IL-1α (R & D Systems), and IL-8 (CXCL8) (eBioscience). Nunc Maxisorp ELISA plates (Nalge Nunc International) were used. The limits of sensitivity were 1–2, 3.9, and 7.8 pg/ml for IL-1β, IL-1α, and IL-8, respectively. They were also analyzed by a multiplex antibody bead kit for IL-6, IL-8, and monocyte chemotactic protein-1 (MCP-1; CCL2; Invitrogen). The limits of sensitivity were 3 (IL-6), 3 (IL-8), and 10 pg/ml (MCP-1).
Lactate Dehydrogenase Cytotoxicity Assay
Cytotoxicity was determined by CytoTox 96 assay kit (Promega, Madison, WI) and calculated as follows: % cytotoxicity = 100 × (experimental lactate dehydrogenase (LDH) − spontaneous LDH)/(maximum LDH − spontaneous LDH).
Western Blotting Analysis
The cells were lysed on ice in lysis buffer (10 mm Tris-HCl, pH 7.4, 150 mm NaCl, 5 mm EDTA, 0.2% Nonidet P-40) supplemented with protease inhibitor mixture (Roche Applied Science) and centrifuged at 15,000 × g for 10 min at 4 °C. Protein concentration was determined by Bio-Rad protein assay kit (Bio-Rad).
The lysates were mixed with SDS sample buffer and heated to 90 °C for 5 min. The proteins were separated by electrophoresis on SDS-PAGE gels and transferred onto polyvinylidene difluoride membranes (0.4 μm) in 25 mm Tris, 192 mm glycine, and 20% methanol at 60 V for 1.5 h. The blots were incubated with primary antibodies at 4 °C overnight followed by the incubation with secondary antibodies. The blots were then developed with horseradish peroxidase substrate (West Femto Solution; Pierce) for 5 min at room temperature and analyzed using GelDoc 200 (Bio-Rad).
Immunoprecipitation
The cells were suspended in lysis buffer (50 mm Tris, pH 7.8, 150 mm NaCl, 0.1% Nonidet P-40, 5 mm EDTA) supplemented with protease inhibitor mixture (Roche Applied Science). The lysates were agitated with 5 μg of anti-ASC antibody for 2 h at 4 °C and mixed with protein G-Sepharose beads (20 μl of 50% slurry) overnight at 4 °C on a shaker. The beads were washed three times with lysis buffer, then mixed with SDS sample buffer, and heated to 90 °C for 5 min followed by SDS-PAGE. The samples were blotted onto polyvinylidene difluoride membranes and then probed with antibodies.
siRNA Transfection
siRNA duplexes targeting ASC (Hs_PYCARD_5, CGGGAAGGTCCTGACGGATGA, and Hs_PYCARD_1, CAGCCTGGAACTGGACCTGCA) and scrambled siRNA for nonspecific gene silencing were from Qiagen. Transfection of siRNA duplexes (2 nm) was carried out using HiPerFect transfection reagent (Qiagen) according to the manufacturer's recommendations. After 24 h, the medium was replaced to OptiMEM (300 μl), and the cells were incubated for additional 24 h. The supernatants and cell lysates were collected for ELISA analysis and Western blotting analysis, respectively.
Generation of Melanoma Conditioned Medium and Its Effects on THP-1 Cells
Melanoma conditioned media (MCM) were obtained from supernatants of human melanoma cells after 24 h of cultivation in OptiMEM and centrifuged at 210 × g for 5 min. THP-1 cells, seeded at 80% confluence, were cultured in MCM for 24 h, and the cell lysates were subjected to ELISA analysis.
Chemotaxis Assay
QCMTM Chemotaxis cell migration assay (Millipore, Temecula, CA) was used. 1205Lu MCM was obtained after 24 h of cultivation of 1205Lu cells in serum-free RPMI 1640 and added to serum-free RPMI 1640 in the feeder tray (lower well). THP-1 cells in serum-free RPMI 1640 were placed into migration chamber and cultured for 2 h. The cells that migrated to the underside or through the membrane were mixed with lysis buffer/CyQuant GR dye solution (Millipore), incubated at room temperature for 15 min, and analyzed with a fluorescence plate reader using a 480/520-nm filter set.
Tube Formation Assay
HUVECs suspended in MCM-containing serum-free M199 medium were cultured for 17 h on polymerized growth factor-reduced Matrigel (BD Biosciences, Bedford, MA). They were fixed in 4% (v/v) paraformaldehyde (Fisher) for 10 min at room temperature and visualized under microscope (40× magnification). The number of tube-like structures with closed networks of vessel-like tubes was counted by two observers independently.
Data Analysis
All of the experiments were replicated at least thrice. The results were analyzed by Student's unpaired t test to compare two groups. One-way analyses of variance followed by Student-Newman-Keuls tests were used to compare multiple groups. The data are presented as the means ± S.E., and differences were considered significant if p < 0.05.
RESULTS
Late Stage Human Melanoma Cell Lines Constitutively Synthesize and Secrete IL-1β
We analyzed the lysates and supernatants of nine human melanoma cell lines. Three metastatic (A375, HS294T, and 1205Lu) and one VGP (WM793B) melanoma cell lines were found to synthesize and secrete IL-1β (Fig. 1A). However, IL-1β secretion was not detectable in two VGP (WM278 and WM75) melanoma cell lines despite the presence of intracellular IL-1β. Neither IL-1β synthesis nor secretion was detected in one VGP (WM115) and two RGP (WM35 and WM1552C) melanoma cell lines. LDH levels in the supernatant were low, confirming that the release of IL-1β was not caused by cell death. To explore the molecular mechanisms responsible for secretion of IL-1β in melanoma, we selected two metastatic melanoma cell lines that secrete IL-1β, 1205Lu and HS294T cells, for further study.
FIGURE 1.
Late stage melanoma but not early stage melanoma constitutively synthesizes and secretes IL-1β. A, mean (± S.E., n = 3 experiments) 24-h levels of intracellular IL-1β (lysates) and secreted IL-1β (supernatants) in various cell lines as described under “Experimental Procedures.” The result of one representative experiment is shown. The error bars are from one experiment done thrice. B, mean (± S.E., n = 3 experiments) 24-h levels of intracellular (upper) and secreted (lower) IL-1β from cell lines as indicated above each graph. The dose-response concentrations of IL-1α stimulation are shown under the horizontal axis. LPS was used at 1.0 μg/ml. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with unstimulated (constitutive) levels indicated as base line. The level of IL-1β (2077 pg/ml) secreted from LPS-stimulated THP-1 cells is shown above the bar.
IL-1 Stimulates IL-1β Synthesis and Release in Human Metastatic Melanoma Cells, 1205Lu and HS294T
We examined the ability of TLR4 ligand (LPS) and IL-1 itself to stimulate IL-1 synthesis and release. Human recombinant IL-1α was used to measure the levels of endogenous IL-1β. PMA-primed THP-1 cells were used as a control. 1205Lu, HS 294T, and THP-1 cells synthesized and secreted IL-1β spontaneously, and the synthesis and secretion were significantly enhanced by the stimulation (Fig. 1B). LDH levels revealed that increased IL-1β release was not the result of increased cell death. For melanoma cells, IL-1α was more effective as a stimulant of IL-1β compared with LPS, whereas LPS was more effective for monocytic cells.
IL-1β-secreting Melanoma Cells Contain Components of an Active Inflammasome
To determine whether the inflammasome pathway is utilized in IL-1β secretion mechanisms in human melanoma cells, we first examined the expression of inflammasome components. Western blotting analysis revealed the expression of each component of the inflammasome in 1205Lu and HS294T cells (Fig. 2A). When stimulated with IL-1α, the synthesis of pro-IL-1β (31-kDa form) was increased. However, the levels of the inflammasome components were unchanged, suggesting that 1205Lu and HS294T cells contain constitutive inflammasome components without additional stimulation.
FIGURE 2.
Inflammasome components are expressed, assembled, and activated in late stage human melanoma cell lines. A, Western blot (WB) analysis of inflammasome components (ASC, NALP1, and NALP3) and IL-1β in THP-1, 1205Lu, and HS294T cells. THP-1 cells were primed with PMA and stimulated for 24 h with IL-1α (10 ng/ml) or LPS (1 μg/ml). Melanoma cells were unstimulated or stimulated for 24 h with IL-1α (10 ng/ml), and the lysates were prepared. The data shown are representative of three separate experiments. B, THP-1, 1205Lu, and HS294T cells were incubated for 24 h in the absence or presence of LPS (1 μg/ml) or IL-1α (10 ng/ml). The cell lysates were immunoprecipitated (IP) with anti-ASC antibody (see “Experimental Procedures”) and then subjected to SDS-PAGE and probed with anti-NALP3 antibody. Western blotting with anti-ASC confirmed the presence of ASC in the immunoprecipitates. C, Western blot analysis of THP-1, 1205Lu, and HS294T cells. Unstimulated constitutive levels are shown in the left lane, whereas the right lane is after 24 h in the presence of LPS (1 μg/ml) or IL-1α (10 ng/ml). The data are one of three representative experiments.
Inflammasome Is Assembled and Activated in IL-1β-secreting Melanoma Cells
ASC is required for NALP3 activation of caspase-1. To further explore the ASC interaction with NALP3, we immunoprecipitated ASC in cell lysates. PMA-primed THP-1 cells were used as a positive control. Immunoprecipitation revealed co-precipitation of NALP3 with ASC in THP-1, 1205Lu, and HS294T cells (Fig. 2B). NALP3 inflammasome assembly was detected even without IL-1α stimulation, suggesting that inflammasome is assembled constitutively in 1205Lu and HS294T cells.
We then examined the cleavage and activation of caspases in 1205Lu and HS294T cells. Activated (cleaved) fragments of caspase-1 (p20) and caspase-5 (p20) were observed in the lysates of 1205Lu and HS294T cells without IL-1α stimulation (Fig. 2C). These data demonstrate that the inflammasome is assembled and activated constitutively, resulting in activation and cleavage of caspases in IL-1β-secreting human melanoma cells.
Melanoma Inflammasome Is Functional and Regulates IL-1β Secretion
We next used siRNA to silence ASC. Scrambled siRNA served as a control. Knockdown of ASC resulted in 70 and 93% reduction of IL-1β secretion compared with control siRNA in 1205Lu and HS294T cells, respectively (Fig. 3A).
FIGURE 3.
The role of inflammasome in the secretion of IL-1β in human melanoma cells. A, upper panels, 1205Lu and HS294T cells were transfected with siRNA (2 nm) against ASC or scrambled RNA indicated as control. 24 h following transfection, the medium was replaced, and the cells were incubated for additional 24 h. The lysates were subjected to Western blotting with anti-ASC antibody. As shown, staining for β-actin was used to control loading. Lower panels, mean (± S.E., n = 4) 24-h secretion of constitutive IL-1β levels was measured in supernatants of the transfectants. The error bars are from one experiment done in quadruplicates, and the experiments were replicated thrice. ***, p < 0.001 compared with the scrambled control. B, 1205Lu and HS294T cells were treated with Z-VAD (2 μm), Z-YVAD (2 μm), Z-WHED (2 μm), or Me2SO control for 30 min, followed by stimulation with IL-1α (10 ng/ml). Cultures without either stimulant are indicated as base line. The data are shown as the means ± S.E. (n = 4). The error bars are from one experiment done in quadruplicate, and the experiments were replicated in triplicate. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with the control (no caspase inhibitors).
To investigate whether IL-1β secretion from melanoma cells requires active caspase-1 and -5, caspase inhibitors were used in cultures of unstimulated as well as stimulated cells. As shown in Fig. 3B, spontaneous and stimulated secretion of IL-1β were reduced by the pan-caspase inhibitor (Z-VAD), caspase-1, 4 inhibitor (Z-YVAD), or caspase-1, 4, 5 inhibitor (Z-WHED). Slight cell death was observed at base line and after caspase inhibition in these cells. These data indicate that inflammatory caspases (caspase-1 and -5) are necessary for releasing IL-1β in human melanoma cells.
Melanoma-derived IL-1β Is Biologically Active Inducing Production and Secretion of Other Cytokines and Chemokines from Melanoma Cells
Because both inactive IL-1β (uncleaved pro-IL-1β) and active (cleaved) IL-1β can be detected by IL-1β ELISA, we determined whether IL-1β secreted from melanoma cells is biologically functional. IL-1Ra was used to block the IL-1R, and as shown in Fig. 4A, there was a reduction of IL-1β synthesis (95% reduction) and release (79% reduction) in 1205Lu cells. These data suggest that active IL-1R signaling is responsible for constitutive IL-1β synthesis and release from melanoma cells. We also tested whether IL-1R signaling affects other cytokines/chemokines known to be associated with melanoma progression (30). When treated with IL-1Ra, 1205Lu cells produced and secreted 90% less IL-6, IL-8, and MCP-1.
FIGURE 4.
Secreted products of melanoma cells induce production and secretion of IL-6, IL-8, and MCP-1 in human melanoma cells and enhance macrophage chemotaxis and angiogenesis in vitro. A, mean (± S.E., n = 3) 24-h levels of intracellular and secreted IL-1β, IL-6, IL-8, and MCP-1 from unstimulated 1205Lu cells in the absence (−) or presence (+) of IL-1Ra (10 μg/ml). The error bars are from one experiment done in triplicate, and the experiments were replicated thrice. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with cells incubated without IL-1Ra. B, 1205Lu MCM (see “Experimental Procedures”) was added to THP-1 cells. After 24 h, the levels of intracellular IL-1β and IL-1α were measured in the lysates. The effect of IL-1Ra (10 μg/ml) present during the generation of the MCM is shown as MCM/IL-1Ra, and the effect of IL-1Ra added after the generation of MCM is shown as MCM+IL-1Ra. The data are shown as the means ± S.E. (n = 3). The error bars are from one experiment done in triplicate, and the experiments were replicated thrice. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with MCM without IL-1Ra. C, THP-1 migration to 1205Lu MCM. The effects of Z-VAD (2 μm), Z-YVAD (2 μm), and IL-1Ra (10 μg/ml) present during the generation of the MCM are shown. The data are presented as the means ± S.E. migration (n = 3) (see “Experimental Procedures”). The error bars are from one experiment done in triplicate, and the experiments were replicated thrice. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with MCM without inhibitors. D, left panel, mean (± S.E., n = 3) IL-8 level in 1205Lu MCM. The effects of Z-VAD (2 μm), Z-YVAD (2 μm), and IL-1Ra (10 μg/ml) present during the generation of the MCM are shown. The error bars are from one experiment done in triplicate, and the experiments were replicated thrice. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with MCM without inhibitors. Center panel, HUVECs were cultured on Matrigel for 17 h with medium only (control), 1205Lu MCM (MCM), and 1205Lu MCM generated in the presence of Z-VAD (2 μm) (MCM/Z-VAD) or IL-1Ra (10 μg/ml) (MCM/IL-1Ra). Tube formation was visualized under a microscope. Bar, 1 mm. Each experiment was performed in sextuplicate, and the experiments were replicated thrice. Representative pictures are shown. Right panel, tube formation by HUVECs incubated with 1205Lu MCM. The effects of Z-VAD (2 μm) and IL-1Ra (10 μg/ml) present during the generation of the MCM are shown. The data are presented as the means ± S.E. tube formation (n = 6). The error bars are from one experiment done in sextuplicate, and the experiments were replicated thrice. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with MCM without inhibitors.
Melanoma-derived IL-1β and Its Downstream Mediators Induce IL-1 Production from Macrophages, Mediate Macrophage Recruitment, and Enhance Angiogenesis in Vitro
To define the biological role of melanoma-derived IL-1β and the downstream mediators induced by IL-1β in tumor microenvironment, we generated supernatants (MCM) of 1205Lu cells after 24 h of cultivation. MCM significantly enhanced the production of IL-1β (2.7-fold) and IL-1α (6.9-fold) in THP-1 cells (Fig. 4B). Whereas pretreating 1205Lu cells with IL-1Ra (MCM/IL-1Ra) abolished this effect, adding IL-1Ra to MCM (MCM+IL-1Ra) attenuated but did not abolish the effect, suggesting that not only melanoma-derived IL-1 itself but also the downstream mediators induced by IL-1 mediate the paracrine effect.
We then examined the effect of melanoma-derived IL-1β and its mediators on macrophage chemotaxis. Whereas 1205Lu MCM enhanced chemotaxis of THP-1 cells 2.7-fold, the effect was abolished by pretreating 1205Lu cells with caspase inhibitors (Z-VAD or Z-YVAD) or IL-1R blockade (Fig. 4C). Adding caspase inhibitors or IL-1Ra into MCM showed no direct inhibitory effects on chemotaxis (data not shown).
1205Lu cells secreted IL-8, an enhancer and stimulator of angiogenesis (31, 32). When 1205Lu cells were treated with caspase inhibitors or IL-1Ra, the release of IL-8 was significantly inhibited (55% inhibition by caspase inhibitors and 91% inhibition by IL-1Ra) (Fig. 4D, left panel). HUVECs were also used to determine the effect of MCM on angiogenesis in vitro. Compared with medium alone (control), 1205Lu MCM induced 1.8-fold more tubular structures formed by HUVECs (Fig. 4D, center and right panels). However, tube formation was reduced when 1205Lu cells were pretreated with caspase inhibitors or IL-1R blockade (Fig. 4D, center and right panels). Taken together, these data indicate the biological function of melanoma-derived IL-1β in a paracrine fashion.
IL-1 Stimulates IL-1β Release in Intermediate Stage Human Melanoma Cell Lines
Because early and intermediate stage melanoma cell lines did not secrete IL-1β at base line, we examined the ability of LPS and IL-1 to induce IL-1 release from these cells. The secretion of IL-1β was induced by IL-1α in three VGP melanoma cell lines (WM278, WM75, and WM115) that did not secrete IL-1β at base line (Table 1). On the contrary, the secretion was not induced by IL-1α in RGP melanoma cell lines (WM35 and WM1552C), despite the induction of IL-1β synthesis. LPS showed little or minimal effects on IL-1β synthesis and secretion in melanoma cell lines (data not shown). LDH levels revealed that increased IL-1β release was not the result of increased cell death.
TABLE 1.
Levels of intracellular and secreted IL-1β in human melanoma cell lines
The mean (n = 3 experiments) 24-h levels of intracellular (IC) and secreted (S) IL-1β from cell lines in the absence (at base line) or presence of IL-1α (10 ng/ml) stimulation are shown.
Early and Intermediate Stage Human Melanoma Cell Lines Contain Components of an Active Inflammasome
Because IL-1β secretion was induced by IL-1α in VGP but not RGP melanoma cell lines, we examined the expression of inflammasome components in WM35, an RGP melanoma cell line, and WM115, a VGP melanoma cell line, both of which had undetectable IL-1β synthesis and secretion at base line. Western blotting analysis revealed the expression of inflammasome components in both WM35 and WM115 cells (Fig. 5A). When stimulated with IL-1α, the production of pro-IL-1β (31-kDa form) was induced in WM115 cells, whereas levels of the inflammasome components were unchanged in both cells. Immunoprecipitation revealed co-precipitation of NALP3 with ASC in WM35 and WM115 cells (Fig. 5B). Western blotting of lysates of WM35 and WM115 cells revealed the p20 cleavage products of both caspase-1 and caspase-5 in the absence of IL-1α stimulation (Fig. 5C).
FIGURE 5.
Inflammasome components are expressed, assembled, and activated in early and intermediate stage human melanoma cell lines. A, Western blot (WB) analysis of inflammasome components (ASC, NALP1, and NALP3) and IL-1β in WM35 and WM115 cells. The cells were unstimulated or stimulated for 24 h with IL-1α (10 ng/ml), and the lysates were prepared. The data shown are representative of three separate experiments. B, cell lysates of WM35, WM115, and HS294T cells were immunoprecipitated (IP) with anti-ASC antibody (see “Experimental Procedures”), then subjected to SDS-PAGE, and probed with anti-NALP3 antibody. Western blotting with anti-ASC confirmed the presence of ASC in the immunoprecipitates. C, Western blot analysis of WM35 and WM115 cells. The unstimulated constitutive levels are shown in the left lane, whereas the right lane is after 24 h in the presence of IL-1α (10 ng/ml). The data are one of three representative experiments.
Despite the presence of active NALP3, in early stage melanoma cells (WM35 and WM1552C cells), we did not observe secretion of IL-1β after stimulation with IL-1α (Table 1). Therefore, these cells were stimulated with the combination of IL-1α plus co-stimulant, the nucleotide oligomerization domain 2 activator muramyl dipeptide. The co-stimulation increased the synthesis of the IL-1β precursor production (1.8-fold increase in WM1552C and 1.5-fold increase in WM35) and induced secretion of IL-1β (data not shown). LDH levels in the supernatants were low, confirming that increased IL-1β release was not due to cell death. The data suggest that unlike late stage melanoma, the constitutive NALP3 inflammasome in early stage melanoma requires stimulation with IL-1 plus a second activator to function for the secretion of IL-1β.
Tumor Cells from Clinical Metastatic Melanoma Constitutively Synthesize and Secrete IL-1β
We analyzed IL-1β synthesis and secretion in clinical melanoma tumors. Fifteen surgically obtained human melanoma tumor specimens (five primary and ten metastatic melanoma tumors) were formalin-fixed and paraffin-embedded. Whereas none of the five primary lesions stained for IL-1β, tumor cells from eight of ten metastatic melanomas stained positively for IL-1β (Fig. 6, A and B). Three metastatic melanoma specimens revealed diffuse IL-1β staining in most tumor cells (Fig. 6A, center panel), whereas five metastatic melanoma specimens showed positive staining in 10–30% tumor cells (Fig. 6A, right panel).
FIGURE 6.
Clinical metastatic melanoma specimens show constitutive secretion of IL-1β and activation of caspases. A, immunohistochemistry of representative clinical melanoma sections. Sample 1 is from primary melanoma, whereas samples 6 and 7 are from metastatic melanoma. Bar, 40 μm. B, summary of the staining. C, mean (± S.E., n = 3 experiments) 24-h levels of intracellular IL-1β (lysates) and secreted IL-1β (supernatants) in tumor cells from primary melanoma (sample 1) or metastatic melanoma (samples 2, 4, 6, and 7). The result of one representative experiment is shown. The error bars are from one experiment done in triplicate. D, Western blot analysis of caspases-1 and -5 from human metastatic melanoma tumor cells (tumors 2 and 6). Melanoma cells were unstimulated, and the lysates were prepared. The data shown are representative of three separate experiments.
We then analyzed the lysates and supernatants of human melanoma tumors. Although three primary melanoma specimens were obtained for the analysis, only one primary melanoma tumor (sample 1) yielded enough cells for further ELISA analysis. Four other samples were obtained from metastatic melanoma tumors (samples 2, 4, 6, and 7). Whereas IL-1β synthesis and secretion were detected in all four metastatic melanoma cells, they were barely detectable in the sample 1 primary melanoma cells (Fig. 6C).
Caspases Are Constitutively Activated in Tumor Cells from Clinical Metastatic Melanoma
To explore the molecular mechanisms responsible for the secretion of IL-1β in clinical metastatic melanoma specimens, we selected two metastatic tumors, samples 2 and 6, for further study. Western blotting analysis revealed activated (cleaved) fragments of caspase-1 (p20) and caspase-5 (p20) in the lysates of human metastatic tumor cells (samples 2 and 6) in the absence of exogenous stimuli (Fig. 6D). These results are in accord with the data from human melanoma cell lines (Figs. 1A and 2C) and confirm our conclusion that constitutively activated caspases mediate secretion of IL-1β in human metastatic melanoma cells.
DISCUSSION
In addition to macrophages, other cell types can utilize inflammasomes to activate and secrete IL-1β. These include keratinocytes, neurons, and mast cells (33–36). However, to the best of our knowledge, no report exists on the expression and function of inflammasomes in melanoma cells. We report here the presence of functional inflammasome as a mechanism of IL-1β secretion in human melanoma cells. Furthermore, we provide evidence that late stage human melanoma cells exhibit a feature of autoinflammatory diseases caused by the constitutive activation of the inflammasome and IL-1R signaling. This is in contrast to human blood monocytes, which do require stimulation by either TLR or IL-1 itself. Therefore, the clinical aggressiveness of late stage human melanoma may be, in part, due to the constitutive secretion of active IL-1β and its downstream inflammatory gene products such as chemokines and angiogenic factors.
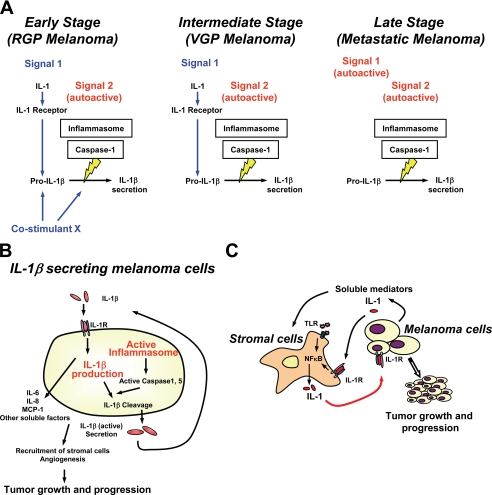
Early studies of inflammasome using unprimed THP-1 cells and mouse peritoneal macrophages show that two distinct stimuli are necessary for the activation and secretion of IL-1β: the first stimulus (Signal 1) to induce transcription and translation of IL-1β and the second (Signal 2) to activate caspase-1 and process IL-1β (37–39). However, our observation reveals that late stage melanoma cells spontaneously secrete bioactive IL-1β in the absence of exogenous stimuli. Recently, Netea et al. (40) demonstrated that IL-1β processing is differentially regulated in monocytes and macrophages by showing that human blood monocytes but not macrophages release IL-1β after a single stimulation with TLR ligands because of constitutively activated caspase-1. This result is in concordance with the present results of human melanoma cells. Constitutively activated caspase-1 (p20) and caspase-5 (p20) were observed in the lysates of all human melanoma cells without additional stimulation. Immunoprecipitation demonstrated constitutive assembly of the NALP3 inflammasome, suggesting that human melanoma cells contain constitutively active inflammasome (autoactive Signal 2). We also observed that pro-IL-1β was produced without stimulation in late stage melanoma cells. Whereas the synthesis was greater upon exposure to exogenous IL-1α, the activity was reduced by blocking IL-1R signaling with IL-1Ra, suggesting that these melanoma cells constitutively transcribe and translate IL-1β through IL-1R signaling (autoactive Signal 1). NF-κB aberrations have been detected in human melanoma cells (41). Activated NF-κB induces cytokines including IL-1, and IL-1R signaling by secreted IL-1 can further activate NF-κB, resulting in the positive feedback loop. We have also observed imbalanced secretion of IL-1β and its anti-inflammatory counterpart, IL-1Ra, in late stage human melanoma cells,4 which may further contribute to autoactivation of IL-1R signaling and NF-κB activity. It appears that secretion of active IL-1β from late stage melanoma cells does not require an exogenous stimulus (Fig. 7A). On the contrary, human melanoma cells from the intermediate stage of the disease require activation of the IL-1R to secrete active IL-1β, and the constitutive NALP3 inflammasome in early stage melanoma requires stimulation with IL-1 plus a second activator to function for the secretion of IL-1β.
FIGURE 7.
Diagrams representing the role of IL-1β and inflammasome in human melanoma. A, the inflammasome is constitutively activated in human melanoma cells (autoactive Signal 2). Late stage melanoma cells secrete IL-1β without exogenous stimuli via autoactive Signal 1 (activation of IL-1R signaling) and autoactive Signal 2. In contrast, intermediate stage human melanoma cells require Signal 1, and early stage human melanoma cells require further stimulation with Signal 1 and co-stimulant for IL-1β secretion. B, IL-1β released from human melanoma cells is biologically active inducing production and secretion of not only IL-1β itself but also other cytokines and chemokines such as IL-6, IL-8, and MCP-1 from human melanoma cells. Together, IL-1β-secreting human melanoma cells recruit stromal cells and induce angiogenesis, leading to tumor progression. C, IL-1β and its downstream mediators released from late stage human melanoma cells induce IL-1 from stromal cells. IL-1 secreted from stromal cells augments autoinflammatory loop of late stage human melanoma cells. IL-1 secreted from stromal cells can also induce IL-1 secretion from intermediate stage human melanoma cells, triggering an autoinflammatory loop.
Melanoma-derived IL-1β induced secretion of IL-1β itself as well as other downstream mediators known to be associated with melanoma progression (Fig. 7B). IL-6 has two properties relevant to melanoma; whereas IL-6 inhibits growth of melanocytes and early stage melanomas, it augments the growth of advanced melanomas by inducing angiogenesis and activating tumor infiltrates (42). IL-8, a CXC chemokine, recruits neutrophils and endothelial cells, promotes melanoma growth, and enhances angiogenesis (30–32). MCP-1 recruits monocytes/macrophages, NK cells, and subpopulation of T cells and stimulates monocytes to produce IL-1 and IL-6 (30). Together with melanoma-derived IL-1β, these downstream mediators IL-6, IL-8, and MCP-1 induced IL-1 production from macrophages, enhanced macrophage chemotaxis, and promoted in vitro angiogenesis.
In the tumor microenvironment, melanoma cells are surrounded by keratinocytes and/or immune cells. These stromal cells produce and secrete IL-1 by stimulation with pathogen-associated molecular patterns (such as TLR agonists) or danger-associated molecular patterns (such as IL-1). It appears that, in late stage human melanoma cells, melanoma-derived IL-1 induces secretion of IL-1 from stromal cells and augments its paracrine loop to activate NF-κB and further secrete IL-1 from human melanoma cells (Fig. 7C). On the contrary, in intermediate stage human melanoma cells, stromal IL-1 works as an exogenous Signal 1 to induce IL-1 secretion from melanoma cells that do not secrete IL-1 at base line. IL-1-induced IL-1 production and secretion is a general property of autoinflammatory diseases (43) and suggests an autoinflammatory nature of metastatically competent (late stage and intermediate stage) human melanoma cells. It is thus reasonable to speculate that IL-1-mediated inflammation contributes to the development and progression of melanoma. Along these lines, it is important to note that inhibiting inflammatory caspases or adding IL-1Ra to melanoma cells significantly reduces the autocrine and paracrine effects of IL-1 in late stage human melanoma whereby IL-1 is responsible for inflammation in tumor-stromal interaction.
It is unclear why the inflammasome pathway is constitutively active in human melanoma cells. In addition to bacterial pathogen-associated molecular patterns, noninfectious danger-associated molecular patterns can activate inflammasomes (22–25). ATP, monosodium urate crystals, contact sensitizers (34), silica crystals, aluminum hydroxide salt, and amyloid-β fibrils can activate inflammasomes (22–25). NALP1 is a transcriptional target for cAMP-response element-binding protein in myeloid leukemia cells (44), and cAMP-response element-binding protein is up-regulated in metastatic melanoma cells (45). Bcl-2 and Bcl-XL, which are increased with melanoma progression (46), regulate caspase-1 activation by interaction with NALP1 (47). Heat shock protein 90, a marker of melanoma progression (48), has a crucial function for NALP3-mediated inflammation (49). Considering the role of active (secreted) IL-1β in cancer progression, it will be intriguing to identify factors that activate inflammasome pathways in human melanoma.
ASC can trigger apoptosis when overexpressed in some tumor cells, and its inactivation and down-regulation by hypermethylation have been reported in many human cancers including melanoma (21, 50–52). In the current study, down-regulation of ASC was observed in 1205Lu cells compared with THP-1 cells and HS294T cells. However, ASC immunoprecipitation revealed interaction of NALP3 and ASC in 1205Lu cells, suggesting that the low expression of ASC in 1205Lu cells was sufficient to interact with NALP3 to induce IL-1β activation and secretion. ASC associates with multiple pyrin- and CARD-containing proteins including pro-caspase-8, IKKα and IKKβ (51, 52). Although the level of ASC expressed in 1205Lu and HS 294T cells varied, the amount of NALP3 that was co-precipitated did not vary, suggesting that the interaction between ASC and NALP3 may not be affected by the presence of other partnering proteins in human melanoma cells.
In summary, we demonstrate here that late stage human melanoma cells exhibit a feature of autoinflammatory diseases by spontaneously secreting active IL-1β via constitutive activation of the NALP3 inflammasome and IL-1R signaling. Because tumor-derived IL-1β is associated with tumor growth, immunosuppression, and chemoresistance (8, 13, 15), constitutive activation of inflammasome pathway and IL-1R signaling may indicate a poor prognosis. We also demonstrate that the secretion of IL-1β from human melanoma cells was reduced by inhibiting inflammasome activity using caspase inhibitors or knocking down of ASC or by using the IL-1Ra. Blocking IL-1 synthesis, secretion, and/or activity may be a monotherapy option or in combination with other treatments in melanoma. Given the safety profile and clinical availability of the IL-1 blockade such as IL-1Ra (53), anti-IL-1β monoclonal antibody (54), the IL-1 Trap (55), and oral caspase-1 inhibitor (56), further evaluation of the mechanisms of IL-1 release from tumors and the use of blockade is warranted for treating human cancer.
Acknowledgments
We thank William A. Robinson and Steven Robinson for providing human melanoma tissue specimen for our studies and Carl K. Edwards and Yiqun G. Shellman (University of Colorado Denver) for helpful suggestions.
This work was supported, in whole or in part, by National Institutes of Health Grants CA125833 (to M. F.) and AI-15614 (to C. A. D.). This work was also supported by grants from the Dermatology Foundation (to M. F.) and the Tadamitsu Cancer Research Fund (to M. F.).
M. Okamoto, C. A. Dinarello, and M. Fujita, unpublished observations.
- IL
- interleukin
- CARD
- caspase recruitment domain
- ELISA
- enzyme-linked immunosorbent assay
- HUVEC
- human umbilical vein endothelial cells
- IL-1R
- IL-1 receptor
- LPS
- lipopolysaccharide
- MCM
- melanoma conditioned media
- MCP-1
- monocyte chemotactic protein-1
- NLR
- nucleotide oligomerization domain-like receptor
- PMA
- phorbol 12-myristate13-acetate
- RGP
- radial growth phase
- TLR
- Toll-like receptor
- VGP
- vertical growth phase
- siRNA
- small interfering RNA
- Z
- benzyloxycarbonyl
- FMK
- fluoromethyl ketone
- LDH
- lactate dehydrogenase.
REFERENCES
- 1.Ostrand-Rosenberg S., Sinha P. (2009) J. Immunol. 182, 4499–4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balkwill F., Mantovani A. (2001) Lancet 357, 539–545 [DOI] [PubMed] [Google Scholar]
- 3.Balkwill F., Coussens L. M. (2004) Nature 431, 405–406 [DOI] [PubMed] [Google Scholar]
- 4.Dinarello C. A. (2009) Annu Rev. Immunol. 27, 519–550 [DOI] [PubMed] [Google Scholar]
- 5.Paugh B. S., Bryan L., Paugh S. W., Wilczynska K. M., Alvarez S. M., Singh S. K., Kapitonov D., Rokita H., Wright S., Griswold-Prenner I., Milstien S., Spiegel S., Kordula T. (2009) J. Biol. Chem. 284, 3408–3417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu T., Tian L., Han Y., Vogelbaum M., Stark G. R. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 4365–4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Müerköster S. S., Lust J., Arlt A., Häsler R., Witt M., Sebens T., Schreiber S., Fölsch U. R., Schäfer H. (2006) Oncogene 25, 3973–3981 [DOI] [PubMed] [Google Scholar]
- 8.Arlt A., Vorndamm J., Müerköster S., Yu H., Schmidt W. E., Fölsch U. R., Schäfer H. (2002) Cancer Res. 62, 910–916 [PubMed] [Google Scholar]
- 9.Jin L., Yuan R. Q., Fuchs A., Yao Y., Joseph A., Schwall R., Schnitt S. J., Guida A., Hastings H. M., Andres J., Turkel G., Polverini P. J., Goldberg I. D., Rosen E. M. (1997) Cancer 80, 421–434 [DOI] [PubMed] [Google Scholar]
- 10.Elaraj D. M., Weinreich D. M., Varghese S., Puhlmann M., Hewitt S. M., Carroll N. M., Feldman E. D., Turner E. M., Alexander H. R. (2006) Clin Cancer Res. 12, 1088–1096 [DOI] [PubMed] [Google Scholar]
- 11.Lewis A. M., Varghese S., Xu H., Alexander H. R. (2006) J. Transl. Med. 4, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J., Richmond A. (2001) Cancer Res. 61, 4901–4909 [PubMed] [Google Scholar]
- 13.Müerköster S., Wegehenkel K., Arlt A., Witt M., Sipos B., Kruse M. L., Sebens T., Klöppel G., Kalthoff H., Fölsch U. R., Schäfer H. (2004) Cancer Res. 64, 1331–1337 [DOI] [PubMed] [Google Scholar]
- 14.Sawai H., Okada Y., Funahashi H., Matsuo Y., Takahashi H., Takeyama H., Manabe T. (2006) BMC Cell Biol. 7, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song X., Krelin Y., Dvorkin T., Bjorkdahl O., Segal S., Dinarello C. A., Voronov E., Apte R. N. (2005) J. Immunol. 175, 8200–8208 [DOI] [PubMed] [Google Scholar]
- 16.Voronov E., Shouval D. S., Krelin Y., Cagnano E., Benharroch D., Iwakura Y., Dinarello C. A., Apte R. N. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 2645–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yano S., Nokihara H., Yamamoto A., Goto H., Ogawa H., Kanematsu T., Miki T., Uehara H., Saijo Y., Nukiwa T., Sone S. (2003) Cancer Sci. 94, 244–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vidal-Vanaclocha F., Alvarez A., Asumendi A., Urcelay B., Tonino P., Dinarello C. A. (1996) J. Natl. Cancer Inst. 88, 198–205 [DOI] [PubMed] [Google Scholar]
- 19.Vidal-Vanaclocha F., Amézaga C., Asumendi A., Kaplanski G., Dinarello C. A. (1994) Cancer Res. 54, 2667–2672 [PubMed] [Google Scholar]
- 20.Dinarello C. A. (2005) J. Exp. Med. 201, 1355–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinon F., Burns K., Tschopp J. (2002) Mol. Cell 10, 417–426 [DOI] [PubMed] [Google Scholar]
- 22.Tschopp J., Martinon F., Burns K. (2003) Nat. Rev. Mol. Cell Biol. 4, 95–104 [DOI] [PubMed] [Google Scholar]
- 23.Ogura Y., Sutterwala F. S., Flavell R. A. (2006) Cell 126, 659–662 [DOI] [PubMed] [Google Scholar]
- 24.Mariathasan S., Monack D. M. (2007) Nat. Rev. Immunol. 7, 31–40 [DOI] [PubMed] [Google Scholar]
- 25.Franchi L., Eigenbrod T., Muñoz-Planillo R., Nuñez G. (2009) Nat. Immunol. 10, 241–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lachmann H. J., Lowe P., Felix S. D., Rordorf C., Leslie K., Madhoo S., Wittkowski H., Bek S., Hartmann N., Bosset S., Hawkins P. N., Jung T. (2009) J. Exp. Med. 206, 1029–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feldmann J., Prieur A. M., Quartier P., Berquin P., Certain S., Cortis E., Teillac-Hamel D., Fischer A., de Saint Basile G. (2002) Am. J. Hum. Genet. 71, 198–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aksentijevich I., Nowak M., Mallah M., Chae J. J., Watford W. T., Hofmann S. R., Stein L., Russo R., Goldsmith D., Dent P., Rosenberg H. F., Austin F., Remmers E. F., Balow J. E., Jr., Rosenzweig S., Komarow H., Shoham N. G., Wood G., Jones J., Mangra N., Carrero H., Adams B. S., Moore T. L., Schikler K., Hoffman H., Lovell D. J., Lipnick R., Barron K., O'Shea J. J., Kastner D. L., Goldbach-Mansky R. (2002) Arthritis Rheum. 46, 3340–3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldbach-Mansky R., Dailey N. J., Canna S. W., Gelabert A., Jones J., Rubin B. I., Kim H. J., Brewer C., Zalewski C., Wiggs E., Hill S., Turner M. L., Karp B. I., Aksentijevich I., Pucino F., Penzak S. R., Haverkamp M. H., Stein L., Adams B. S., Moore T. L., Fuhlbrigge R. C., Shaham B., Jarvis J. N., O'Neil K., Vehe R. K., Beitz L. O., Gardner G., Hannan W. P., Warren R. W., Horn W., Cole J. L., Paul S. M., Hawkins P. N., Pham T. H., Snyder C., Wesley R. A., Hoffmann S. C., Holland S. M., Butman J. A., Kastner D. L. (2006) N. Engl. J. Med. 355, 581–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raman D., Baugher P. J., Thu Y. M., Richmond A. (2007) Cancer Lett. 256, 137–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li A., Dubey S., Varney M. L., Dave B. J., Singh R. K. (2003) J. Immunol. 170, 3369–3376 [DOI] [PubMed] [Google Scholar]
- 32.Bar-Eli M. (1999) Pathobiology 67, 12–18 [DOI] [PubMed] [Google Scholar]
- 33.Feldmeyer L., Keller M., Niklaus G., Hohl D., Werner S., Beer H. D. (2007) Curr. Biol. 17, 1140–1145 [DOI] [PubMed] [Google Scholar]
- 34.Watanabe H., Gaide O., Pétrilli V., Martinon F., Contassot E., Roques S., Kummer J. A., Tschopp J., French L. E. (2007) J. Invest. Dermatol. 127, 1956–1963 [DOI] [PubMed] [Google Scholar]
- 35.de Rivero Vaccari J. P., Lotocki G., Marcillo A. E., Dietrich W. D., Keane R. W. (2008) J. Neurosci. 28, 3404–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura Y., Kambe N., Saito M., Nishikomori R., Kim Y. G., Murakami M., Núñez G., Matsue H. (2009) J. Exp. Med. 206, 1037–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinon F., Agostini L., Meylan E., Tschopp J. (2004) Curr. Biol. 14, 1929–1934 [DOI] [PubMed] [Google Scholar]
- 38.Mariathasan S., Weiss D. S., Newton K., McBride J., O'Rourke K., Roose-Girma M., Lee W. P., Weinrauch Y., Monack D. M., Dixit V. M. (2006) Nature 440, 228–232 [DOI] [PubMed] [Google Scholar]
- 39.Kanneganti T. D., Lamkanfi M., Kim Y. G., Chen G., Park J. H., Franchi L., Vandenabeele P., Núñez G. (2007) Immunity 26, 433–443 [DOI] [PubMed] [Google Scholar]
- 40.Netea M. G., Nold-Petry C. A., Nold M. F., Joosten L. A., Opitz B., van der Meer J. H., van de Veerdonk F. L., Ferwerda G., Heinhuis B., Devesa I., Funk C. J., Mason R. J., Kullberg B. J., Rubartelli A., van der Meer J. W., Dinarello C. A. (2009) Blood 113, 2324–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amiri K. I., Richmond A. (2005) Cancer Metastasis Rev. 24, 301–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun W. H., Kreisle R. A., Phillips A. W., Ershler W. B. (1992) Cancer Res. 52, 5412–5415 [PubMed] [Google Scholar]
- 43.Dinarello C. A. (2009) N. Engl. J. Med. 360, 2467–2470 [DOI] [PubMed] [Google Scholar]
- 44.Sanz C., Calasanz M. J., Andreu E., Richard C., Prosper F., Fernandez-Luna J. L. (2004) Biochem. J. 384, 281–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jean D., Bar-Eli M. (2000) Mol. Cell Biochem. 212, 19–28 [PubMed] [Google Scholar]
- 46.Leiter U., Schmid R. M., Kaskel P., Peter R. U., Krähn G. (2000) Arch. Dermatol. Res. 292, 225–232 [DOI] [PubMed] [Google Scholar]
- 47.Bruey J. M., Bruey-Sedano N., Luciano F., Zhai D., Balpai R., Xu C., Kress C. L., Bailly-Maitre B., Li X., Osterman A., Matsuzawa S., Terskikh A. V., Faustin B., Reed J. C. (2007) Cell 129, 45–56 [DOI] [PubMed] [Google Scholar]
- 48.McCarthy M. M., Pick E., Kluger Y., Gould-Rothberg B., Lazova R., Camp R. L., Rimm D. L., Kluger H. M. (2008) Ann. Oncol. 19, 590–594 [DOI] [PubMed] [Google Scholar]
- 49.Mayor A., Martinon F., De Smedt T., Pétrilli V., Tschopp J. (2007) Nat. Immunol. 8, 497–503 [DOI] [PubMed] [Google Scholar]
- 50.Masumoto J., Taniguchi S., Ayukawa K., Sarvotham H., Kishino T., Niikawa N., Hidaka E., Katsuyama T., Higuchi T., Sagara J. (1999) J. Biol. Chem. 274, 33835–33838 [DOI] [PubMed] [Google Scholar]
- 51.Stehlik C., Fiorentino L., Dorfleutner A., Bruey J. M., Ariza E. M., Sagara J., Reed J. C. (2002) J. Exp. Med. 196, 1605–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McConnell B. B., Vertino P. M. (2004) Apoptosis 9, 5–18 [DOI] [PubMed] [Google Scholar]
- 53.den Broeder A. A., de Jong E., Franssen M. J., Jeurissen M. E., Flendrie M., van den Hoogen F. H. (2006) Ann. Rheum. Dis. 65, 760–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lachmann H. J., Kone-Paut I., Kuemmerle-Deschner J. B., Leslie K. S., Hachulla E., Quartier P., Gitton X., Widmer A., Patel N., Hawkins P. N. (2009) N. Engl. J. Med. 360, 2416–2425 [DOI] [PubMed] [Google Scholar]
- 55.Hoffman H. M., Throne M. L., Amar N. J., Sebai M., Kivitz A. J., Kavanaugh A., Weinstein S. P., Belomestnov P., Yancopoulos G. D., Stahl N., Mellis S. J. (2008) Arthritis Rheum. 58, 2443–2452 [DOI] [PubMed] [Google Scholar]
- 56.Stack J. H., Beaumont K., Larsen P. D., Straley K. S., Henkel G. W., Randle J. C., Hoffman H. M. (2005) J. Immunol. 175, 2630–2634 [DOI] [PubMed] [Google Scholar]