Abstract
>Background
Atherosclerosis is a chronic inflammatory disease of the arterial vessel wall. The A2A receptor (A2AR) plays a central role in many anti-inflammatory effects of adenosine. However, the role of A2AR in atherosclerosis is not clear.
>Methods and Results
The knockout of A2AR in apolipoprotein E–deficient (Apoe−/−/A2AR−/−) mice led to an increase in body weight and levels of blood cholesterol and proinflammatory cytokines, as well as the inflammation status of atherosclerotic lesions. Unexpectedly, Apoe−/−/A2AR−/− mice developed smaller lesions, as did chimeric Apoe−/− mice lacking A2AR in bone-marrow-derived cells (BMDCs). The lesions of those mice exhibited a low density of foam cells and the homing ability of A2AR-deficient monocytes did not change. Increased foam cell apoptosis was detected in atherosclerotic lesions of Apoe−/−/A2AR−/− mice. In the absence of A2AR, macrophages incubated with oxidized LDL or in vivo-formed foam cells also exhibited increased apoptosis. A2AR deficiency in foam cells resulted in an increase in p38 mitogen-activated protein kinase (MAPK) activity. Inhibition of p38 phosphorylation abrogated the increased apoptosis of A2AR-deficient foam cells.
>Conclusion
Inactivation of A2AR, especially in BMDCs, inhibits the formation of atherosclerotic leisons, suggesting that A2AR inactivation may be useful for the treatment of atherosclerosis.
>Introduction
Atherosclerosis is a chronic inflammatory disease of the arterial vessel wall that involves endothelial cells, vascular smooth muscle cells, mononuclear cells, platelets, growth factors, and inflammatory cytokines 1–3. Conditions that increase inflammation also exacerbate atherosclerosis in vivo, and most drugs that improve the clinical outcome of atherosclerosis also inhibit inflammation 2. Therefore, inflammation is considered a therapeutic target in atherosclerosis 2.
Adenosine is an endogenous regulator of inflammation and tissue injury, and most of its anti-inflammatory effects are elicited via A2AR 4. A2AR exists on many inflammatory cells, including neutrophils, monocytes, lymphocytes, macrophages, and platelets 4, 5, and loss of A2AR increases inflammatory responses and tissue damage in vivo 6, 7. In contrast, occupancy of A2AR reduces inflammation and protects tissues from injury 5. Therefore, A2AR is considered as an inflammatory modulator and promising pharmacological target for the treatment of inflammatory disorders.
A2AR plays a complex role in inflammation and tissue injury. In the context of neurological disease, blocking A2AR appears to be beneficial 8. Several A2AR antagonists are being developed to treat neurological disorders, and some of these are even being assessed in clinical trials 9. Notably, many patients with neurodegenerative disease also suffer from vascular disease associated with atherosclerosis. Thus, it is relevant to study whether blocking A2AR also affects atherosclerosis. To date, however there have been no reports on the effects of blocking or knocking out A2AR on atherosclerosis. Therefore, we evaluated whether A2AR deficiency affects atherosclerosis using mice deficient for both A2AR and Apoe (Apoe−/−/A2AR−/−).
>Materials and Methods
>Mice
A2AR−/− mice in C57BL/6J background 10 were bred with apoE−/− (C57BL/6J background) mice to generate Apoe−/−/A2AR−/− mice and their littermate controls. Chimeric mice with or without A2AR in their bone marrow-derived cells were produced by bone marrow transplantation, as described 11. Mice were fed a western diet for 3 months or 6 months and then euthanized for collection of aortas. All animal experiments and care were approved by the University of Minnesota Animal Care and Use Committee, in accordance with AAALAC guidelines.
Blood lipid and leukocyte analysis
Blood lipid was determined via an automated enzymatic technique (Boehringer Mannheim GmbH). Blood leukocytes were quantified using an automated blood cell counter (Hemavet 850FS, CDC Technologies, Oxford, CT).
Measurement of plasma cytokines
Cytokine levels were determined by multiplex assay on the Luminex (Austin, TX) platform using BioPlex software (Bio-Rad, Hercules, CA) and mouse-specific bead sets according to the manufacturer's instructions (R&D Systems, Minneapolis, MN).
Preparation of mouse aortas and quantification of atherosclerosis
Aortas of atherosclerotic mice were collected and processed for oil red O staining using either en face preparation of whole aortas or cross-sections of aortic sinuses 12.
Histological analysis of atherosclerotic lesions
Red oil O staining was performed on frozen sections (5 µm thick) of atherosclerotic aortic sinuses. Using specific antibodies, immunostaining to detect expression of macrophage F4/80 (Accurate, Westbury, NY) and phospho-NF-κB p65 (Cell signaling, Danvers, MA) was performed. Slides were examined under a light microscope (Carl Zeiss, Thornwood, NY), and images were digitized into a Macintosh computer. Samples from ten mice were analyzed per group. Quantification was done by dividing the area of positive staining by the total measured lesion area in digitized images.
TUNEL assays
Thioglycollate-elicited peritoneal macrophages were recovered from wt or A2AR−/−mice, plated in 8-well culture slides (BD, Bedford, MA) at 0.5 × 106 cells/well, and cultured in DMEM/10% fetal bovine serum for 16 h. The cells were then incubated with 100 µg/mL of human oxidized LDL (ox-LDL) (Biomedical Technologies, Stoughton, MA) with or without the p38 inhibitor SB203580 at 20 µM (Calbiochem, La Jolla, CA) for 20 h. Cells were fixed in 4% paraformaldehyde and apoptosis determined using the Dead End Fluorometric TUNEL System (Promega, Madison, WI) following the manufacturer’s instructions. The same TUNEL staining was also conducted on frozen sections of mouse aortic sinuses to examine the apoptotic cells in atherosclerotic lesions.
Real-time PCR
Total RNA from atherosclerotic arteries was extracted using Trizol reagent (Invitrogen, Carlsbad, CA), and cDNA was synthesized using a first-strand cDNA synthesis kit (Fermentas). PCR was performed with a LightCycler 2.0 thermal cycler (Roche, Indianapolis, IN) using SYBR Green as a double-stranded DNA–specific dye. The relative amount of each gene in each sample was estimated by the ΔΔCT method. Supplemental table 4 lists the sequences of primers for cytokines.
Western blotting
Mouse peritoneal macrophages were lysed and transferred to a polyvinylidene fluoride membrane. Antibodies against p38, phospho-p38, caspase-3 and GAPDH (Cell Signaling, Danvers, MA) were applied. The blots were incubated with alkaline phosphatase–conjugated secondary antibodies, developed with a chemifluorescence reagent, and scanned by Storm 860 (GE Healthcare).
Electrophoretic mobility shift assay
Nuclear protein was extracted using NucBuster Protein Extraction kit (Novagen, San Diego, CA). A biotin end-labeled double-stranded oligonucleotide (5′-biotin-GGAGAGTGGGGACTACCCCCTCTGCT-3′) and a non-labeled oligonucleotide containing the NF-κB consensus sequence were incubated with the extracted nuclear protein. The samples were subjected to SDS-PAGE and transferred to a nylon membrane. The biotin-labeled DNA was detected with the LightShift Chemiluminescent Electrophoretic Mobility Shift Assay kit (Pierce, Rockford, IL).
Statistical analysis
Statistical analysis was performed with Instat software (GraphPad). Data are presented as mean ± SEM. Data were analyzed with either a one-way ANOVA followed by a Bonferroni correction post-hoc test or a Student’s t-test to evaluate two-tailed levels of significance. The null hypothesis was rejected at P < 0.05.
Results
Atherosclerosis in Apoe−/−/A2AR−/− mice
To determine the role of A2AR in the development of atherosclerotic lesions in vivo, Apoe−/−/A2AR−/− mice and their littermate Apoe−/− mice were fed a chow diet or western diet for three months. These mice exhibited no differences in blood pressure, number of circulating leukocytes, differential counts, or blood glucose (Suppl. Table 1, 2, 3). The level of blood alanine aminotransferase (ALT) in Apoe−/−/A2AR−/− mice was four time higher than that in Apoe−/− mice on western diet (Suppl. Table 4). The weight of Apoe−/−/A2AR−/− mice fed a Western diet was 23% higher for males and 12% higher for females compared with sex-matched Apoe−/− mice fed the same diet. Total blood cholesterol was 45% higher in male Apoe−/−/A2AR−/− mice and 25% higher in females compared with Apoe−/− mice on both chow and western diets; this increase was due solely to increased LDL cholesterol (Table 1 and Suppl. Table 3). Interestingly, lipid profiles were similar in A2AR−/− and wild type mice on western diet. In A2AR−/− and wild type mice on both chow and western diet, blood IL-6 levels were not detectable. In contrast, blood IL-6 levels were detectable and much higher in Apoe−/−/A2AR−/− than in Apoe−/− mice (25 ± 8.2 versus 20 ± 5.8 pg/mL, as shown in Suppl. Table 5). Despite the higher body weight, blood cholesterol, and proinflammatory cytokine levels, atherosclerotic lesion size in the aortas of Apoe−/−/A2AR−/− mice was decreased by 26% in females and 20% in males compared to Apoe−/− mice (Fig. 1b). In addition, the aortic sinuses displayed much smaller lesions in Apoe−/−/A2AR−/− mice than in Apoe−/− mice (Fig. 1c).
Table 1.
| Genotype | Gender | n | Body weight(g) |
Total cholesterol (mg/dL) |
LDL (mg/dL) |
|---|---|---|---|---|---|
| 3 months WD | |||||
| Apoe−/−/A2AR+/+ | Male | 15 | 34.5±6.2 | 1262.5±197.3 | 1067.4±147.8 |
| Apoe−/−/A2AR−/− | 15 | 40.7±8.0* | 1828.1±203.7* | 1426.6±194.0* | |
| *P=0.012 | *P=0.023 | *P=0.024 | |||
| Apoe−/−/A2AR+/+ | Female | 15 | 25.6±3.2 | 898.8±76.7 | 657.1±82.7 |
| Apoe−/−/A2AR−/− | 15 | 28.7±6.2* | 1115.8±278.1* | 853.9±151.2* | |
| *P=0.021 | *P=0.028 | *P=0.021 | |||
| 6 months WD | |||||
| Apoe−/−/A2AR+/+ | Male | 15 | 31.2±6.1 | 1156.2±146.7 | 957.8±85.6 |
| Apoe−/−/A2AR−/− | 15 | 38.1±10.8* | 1482.4±201.6* | 1243.3±176.4* | |
| *P=0.034 | *P=0.012 | *P=0.017 | |||
| Apoe−/−/A2AR+/+ | Female | 15 | 25.6±6.0 | 670.2±76.4 | 512.7±65.5 |
| Apoe−/−/A2AR−/− | 15 | 28.6±4.9* | 859.2±92.7* | 674.6±81.4* | |
| *P=0.034 | *P=0.023 | *P=0.025 |
Figure 1. A2AR deficiency decreases atherosclerotic lesion formation in Apoe−/− mice.
a and b, Oil red O (ORO) staining of aortas. c and d, ORO and anti-F4/80 staining of aortic sinuses. e, mRNA level of CD68 of aortic sinuses. f and g, Quantitative data of aortic sinuses from chimeric mice.
To examine whether A2AR deficiency could also protect mice from advanced atherosclerosis, Apoe−/−/A2AR−/− mice and their littermate Apoe−/− mice were placed on a Western diet for six months. In accordance with the changes in mice fed a Western diet for three months, Apoe−/−/A2AR−/− mice gained more body weight and had a much higher level of blood total cholesterol than Apoe−/− mice (Table 1). Aortic atherosclerotic lesions in female Apoe−/−/A2AR−/− mice were 51% smaller compared with those in female Apoe−/− mice, and the lesions in male Apoe−/−/A2AR−/− mice were 55% smaller compared with controls (Fig. 1a). These results confirmed the data obtained from mice fed a Western diet for three months, and demonstrated even greater protection against atherosclerosis in Apoe−/−/A2AR−/− mice during a longer period of atherosclerotic challenge.
The cellular components of atherosclerotic lesions in cross-sections of the aortic sinus were also compared. Macrophages and foam cells were mainly located in the cap and shoulders of lesions in Apoe−/− and Apoe−/−/A2AR−/− mice, but the total number of macrophages and foam cells in lesions of Apoe−/−/A2AR−/− mice was greatly diminished (Fig. 1d). This was further supported by the lower levels of mRNA encoding the monocyte marker CD68 in lesions of Apoe−/−/A2AR−/− mice than those of Apoe−/− mice (Fig. 1e).
Atherosclerosis in chimeric mice lacking A2AR and apoE in bone marrow–derived cells (BMDCs)
To determine the influence of leukocyte A2AR in the formation of atherosclerotic lesions, we studied atherosclerosis in Apoe−/− chimeric mice fed a Western diet for three months. Apoe−/− mice lacking A2AR in their BMDCs did not differ from Apoe−/− mice in body weight or blood cholesterol level (data not shown). In the aortic sinuses, a 30% reduction was observed in the average size of lesions in chimeric mice lacking A2AR in their BMDCs compared to that in controls (Fig. 1f), suggesting that protection against atherosclerosis in Apoe−/−/A2AR−/− mice was mainly due to A2AR deficiency in BMDCs.
The presence of macrophages in atherosclerotic lesions of chimeric mice was also assessed; Apoe−/− mice lacking A2AR in their BMDCs demonstrated significantly fewer macrophages in lesions compared with Apoe−/− mice (Fig. 1g).
Inflammatory status of atherosclerotic lesions in Apoe−/−/A2AR−/− mice
Atherosclerosis is a chronic inflammatory disease, and disease progression is usually accompanied by increased inflammation 2. A2AR−/− mice and A2AR-deficient macrophages exhibited increased inflammatory phenotype following inflammatory stimulation 6, 7. Since Apoe−/−/A2AR−/− mice developed small atherosclerotic lesions, we speculated that A2AR-deficient macrophages might react to modified LDL differently from their response to other inflammatory stimuli. To test this possibility, we examined the inflammatory response of A2AR-deficient macrophages to ox-LDL in an in vivo peritonitis model. On the third day of thioglycollate-induced peritonitis, mice were injected intraperitoneally with ox-LDL. Peritoneal macrophages were collected 30 minutes after the ox-LDL injections. As shown by an electrophoretic mobility shift assay, both wt and A2AR-deficient macrophages displayed significant levels of nuclear P65/P50 binding to the NF-κB consensus sequence, indicating activation of the NF-κB pathway in thioglycollate-elicited macrophages. Compared with wt macrophages, A2AR-deficient macrophages showed increased NF-κB activation before and after ox-LDL treatment (Fig. 2a).
Figure 2. A2AR deficiency elevates inflammatory status of atherosclerotic lesions in Apoe−/− mice.
a, Electrophoretic mobility shift assay to assess NF-κB activation induced by ox-LDL in a thioglycollate-induced peritonitis model. b, Immunostaining of phospho-p65 (pP65) and F4/80 of aortic sinuses. c, mRNA expression in atherosclerotic lesions.
To determine the level of NF-κB activation in foam cells present in atherosclerotic lesions of Apoe−/−/A2AR−/− mice, sections of atherosclerotic lesions were immunostained with phosphor-p65-specific antibody. Phosphorylation of p65 is an indicator of NF-κB activation. Among the macrophages/foam cells present in lesions, many more cells demonstrated positive staining for phospho-p65 in lesions of Apoe−/−/A2AR−/− mice than in those of Apoe−/− mice (Fig. 2b). In addition to NF- κB signaling, we also determined the mRNA levels of proinflammatory cytokines in lesions by real-time RT-PCR. The levels of IL-1b and IL-6 mRNA were much higher in atherosclerotic lesions of Apoe−/−/A2AR−/− mice than in those of Apoe−/− mice (Fig. 2c). These results indicate that, in an atherosclerotic environment, A2AR-deficient macrophages exhibited an inflammatory phenotype. Notably, the mRNA level of IL-10, an anti-inflammatory cytokine, was also increased in lesions of Apoe−/−/A2AR−/− mice.
Apoptotic foam cells in atherosclerotic lesions of Apoe−/−/A2AR−/− mice
Apoptosis of macrophages or foam cells during the early stages of atherosclerosis decreases atherosclerosis 13–15. To investigate whether this was the mechanism responsible for suppressed atherosclerosis in Apoe−/−/A2AR−/− mice, we first performed TUNEL-staining to detect apoptotic cells on cross-sections of atherosclerotic lesions. In lesion areas containing F4/80-positive macrophages, many more cells were positive for TUNEL-staining in lesions of Apoe−/−/A2AR−/− mice than in those of Apoe−/− mice (Fig. 3a).
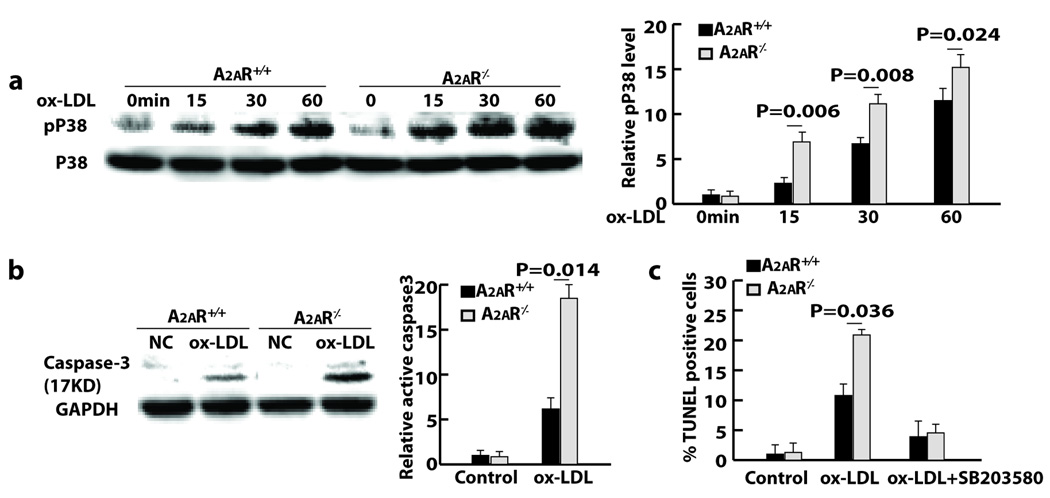
Figure 3. A2AR deficiency increases apoptosis of foam cells.
a, Apoptosis and anti-F4/80 staining of atherosclerotic lesions. b and c, Percentages of apoptotic foam cells isolated from the peritoneal cavities on day 3 after thioglycollate injection. d, Western blot showing the level of active caspase-3 fragment in peritoneal foam cells.
Macrophages in the peritoneal cavities of atherosclerotic mice with thioglycollate-induced peritonitis differentiate into foam cells 16. Using this in vivo foam cell formation model, wt and A2AR-deficient foam cells were generated and assayed by flow cytometry. Among the F4/80-positive foam cells, the percentage of annexin V-positive but PI-negative cells was 12% for foam cells from Apoe−/−/A2AR−/− mice and 5% for foam cells from Apoe−/− mice (Fig. 3b). Similar results were obtained by TUNEL-staining (Fig. 3c). Caspase-3 is a critical executioner of apoptosis, and the cleaved p17 fragment represents its active form. The p17 fragment of caspase-3 was detected in foam cells by western blot. The level of p17 was much higher in A2AR-deficient foam cells than wt cells (Fig. 3d).
Activation of p38 MAPK in A2AR-deficient macrophages
Activation of A2AR increases intracellular cAMP 4, which, in turn, inhibits activation of the intracellular signaling molecule p38 MAPK via the cAMP response element–binding protein–induced dynein light chain 17. In an in vitro assay using isolated peritoneal macrophages, p38 MAPK activation in response to ox-LDL stimulation was much more robust in A2AR-deficient than in wt macrophages (Fig. 4a). Furthermore, the level of ox-LDL-induced active caspase-3 was much higher in A2AR-deficient macrophages than wt macrophages (Fig. 4b). To determine whether p38 activation was a possible mechanism for the increased apoptosis of A2AR-deficient macrophages, A2AR-deficient macrophages were first pretreated with the p38 inhibitor SB203580, followed by incubation with ox-LDL for induction of apoptosis. Incubation with ox-LDL elicited apoptosis in 20% of A2AR-deficient macrophages and 9% of wt macrophages. SB203580 pretreatment decreased ox-LDL-mediated apoptosis in both cases, but this decrease was more pronounced for A2AR-deficient macrophages than wt cells. The percentage of apoptotic A2AR-deficient macrophages was reduced almost to the level measured for wt macrophages, indicating that increased p38 activation is the underlying mechanism for apoptosis of A2AR-deficient macrophages (Fig. 4c).
Figure 4. A2AR deficiency increases p38 activation in macrophages.
a and b, Western blot showing levels of phosphorylated p38 (pP38) and active caspase-3 in peritoneal macrophages after ox-LDL stimulation (20 hours for b). c, Quantitative data of staining showing the effect of p38 activation on ox-LDL-mediated macrophage apoptosis.
Discussion
Previous studies have shown that A2AR deficiency exacerbates inflammatory reactions and induces severe tissue injury 6, 7, and the present work demonstrates that Apoe−/−/A2AR−/− mice had increased body weight, considerable hypercholesterolemia, and increased proinflammatory cytokines in the blood. These data would predict a severe atherosclerotic phenotype in Apoe−/−/A2AR−/− mice. Thus, the observed suppression of atherosclerosis in Apoe−/−/A2AR−/− mice was highly unexpected. The initial data showing decreased atherosclerosis in mice fed a Western diet for three months were surprising, and led us to subsequently assess mice fed a Western diet for six months. A2AR deficiency led to even greater protection against atherosclerosis when mice were provided a Western diet for a longer period. Results from these two animal studies unambiguously support a protective role for A2AR inactivation in atherosclerosis.
The protective role of A2AR deficiency or blockade has mostly been observed in neurological disease models 8. Loss or blockade of A2AR decreases ischemic brain injury and neurotoxicity in models of Parkinson’s disease and Huntington’s disease 10, 18–20. Blocking A2AR-mediated glutamate release from the ischemic and nonischemic cortex and striatum has been proposed as the mechanism for these beneficial effects 21. A recent study found that either global or BMDC-specific A2AR deficiency in mice attenuated infarct volumes in an ischemic brain injury model 22. This protection was associated with a decline in the ischemia-induced expression of several proinflammatory cytokines. Using the same real-time RT-PCR assay, we found that the expression of cytokines in atherosclerotic lesions of Apoe−/−/A2AR−/− mice was higher than that of Apoe−/− mice, indicating that the mechanism for protection against atherosclerosis due to A2AR deficiency differs from that involved in neuroprotection.
A2AR deficiency has adverse effects in most animal models of peripheral organ diseases. A2AR−/− mice exhibit extensive liver damage due to prolonged and enhanced expression of proinflammatory cytokines (such as TNF-α, IL-6, and IL-12) in concanavalin A– or endotoxin-induced septic shock and ischemic liver injury models 6. Additionally, in a renal ischemia reperfusion injury model, plasma creatinine and cytokines are significantly increased in A2AR−/− compared to wt mice 23. In an adenosine deaminase–deficient model of pulmonary inflammation, A2AR deficiency causes enhanced pulmonary leukocyte infiltration and mucin production in the bronchial airways, as well as elevated levels of MCP-1 and CXCL1 24. A2AR-mediated protection may be achieved via suppression of the generation of reactive oxygen species and proinflammatory cytokines in inflammatory cells 4. In line with the above studies, we found that proinflammatory cytokines were increased in the circulating blood and atherosclerotic lesions of Apoe−/−/A2AR−/− mice.
Macrophage phenotype is modulated through adenosine A2AR activation. A2AR agonists synergize toll like receptors to switch macrophages from an M1 (inflammatory) phenotype to an M2 (angiogenic) phenotype 25. Thus, due to lack of A2AR, macrophages in lesions maintain themselves in the M1 phenotype. Indeed, NF–κB activation was enhanced in lesion foam cells of A2AR−/−/apoE−/− mice. In an in vitro assay, A2AR deficient macrophages also exhibited increased NF–κB activation in response to ox-LDL, though ox-LDL may stimulate different receptors compared to minimally modified LDL and the effects of these ligands might discriminate important differences between wild type and A2AR deficient macrophages 26. Nevertheless, results from both in vivo and in vitro setups confirm the inflammatory phenotype of A2AR-deficient macrophages and foam cells under atherosclerotic conditions. Contrary to the general concept that suppression of macrophage inflammatory reactions reduces atherosclerosis, inhibition of NF-κB activity by deletion of IKK2 decreases macrophage inflammatory phenotype, but enlarges atherosclerotic lesions 27. Therefore, enhanced macrophage inflammatory phenotype may not directly lead to an increase of atherosclerotic lesion size.
The size of atherosclerotic lesions is directly related to the number of foam cells within the lesions, which is balanced by monocyte recruitment, macrophage apoptosis, and macrophage emigration from lesions 2. No significant difference was found in monocyte homing ability between wt and A2AR-deficient monocytes (Suppl. Fig. 1). However, the number of macrophages in atherosclerotic lesions of Apoe−/−/A2AR−/− mice was less than that of Apoe−/− mice. This led us to examine whether A2AR deficiency induces macrophage apoptosis in atherosclerotic lesions.
Macrophage or foam cell apoptosis occurs during all stages of atherosclerosis and plays a different role in atherosclerosis depending on the stage at which it occurs 28, 13. During late stages of atherosclerosis, apoptosis contributes to the formation of necrotic cores and to lesion vulnerability 29. However, during the early stages of atherosclerosis, apoptosis decreases the number of foam cells and the size of atherosclerotic lesions 14, 15. In lesions of Apoe−/−/A2AR−/− mice, most apoptoic cells were localized in the subendothelial space, indicating early apoptosis of foam cells. In response to oxLDL treatment, A2AR-deficient macrophages exhibited increased p38 MAPK activation. This may result from a change in signaling associated with intracellular cAMP 4. Elevation of cAMP following A2AR occupancy inhibits activation of p38 via the cAMP response element–binding protein–induced dynein light chain, and p38 activation has been linked to apoptosis 17, 30. A recent study showed that p38 mediates caspase-3 activation and apoptosis in macrophages stimulated with ATP and H2O2. A2AR-deficient macrophages challenged with modified LDL may utilize similar pathways because the p38 inhibitor can inhibit caspase-3 activation and apoptosis 31. We have attempted to elucidate molecular mechanisms underlying the apoptosis of A2AR-deficient macrophages, but we have yet to find a difference in the levels of Bcl-2, Bax, and Bcl-XL between wt and A2AR-deficient macrophages.
Activation of A2AR using agonists dramatically inhibits inflammation and protects against tissue injury. A2AR activation protects against ischemia in the myocardium, kidney, liver, spinal cord, and brain 5. Additionally, administration of A2AR agonists improves survival in mouse models of endotoxemia and sepsis 32, and attenuates inflammation and injury in lipopolysaccharide-induced lung injury 33, diabetic nephropathy 34, and inflammatory bowel disease 35. Recent studies have shown that A2AR agonists inhibit foam cell formation and vascular remodeling after injury 36, 37. It is very likely that A2AR agonists inhibit the formation of atherosclerotic lesions. The anti-atherosclerotic effects of A2AR deficiency do not rule out the potential efficacy of A2AR agonists in the treatment of atherosclerosis.
In summary, our data provide evidence that A2AR inactivation protects against atherosclerosis. A2AR deficiency increases p38 activation in macrophages and foam cells, and this modulation in signaling induces activation of caspase-3. The latter drives foam cells toward apoptosis, thus reducing the size of atherosclerotic lesions. This study suggests that A2AR inactivation represents a new direction for anti-atherosclerotic therapies.
Supplementary Material
Acknowledgments
The authors thank Dr. Anne Marie Weber-Main for her critical review and editing of manuscript drafts.
Sources and Funding
This work was supported by AHA 0430151N, NIH HL78679, and HL080569 grants to Y. Huo.
Footnotes
Disclosures
None.
Reference List
- 1.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 3.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 4.Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Lappas CM, Sullivan GW, Linden J. Adenosine A2A agonists in development for the treatment of inflammation. Expert Opin Investig Drugs. 2005;14:797–806. doi: 10.1517/13543784.14.7.797. [DOI] [PubMed] [Google Scholar]
- 6.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 7.Lukashev D, Ohta A, Apasov S, Chen JF, Sitkovsky M. Cutting edge: Physiologic attenuation of proinflammatory transcription by the Gs protein-coupled A2A adenosine receptor in vivo. J Immunol. 2004;173:21–24. doi: 10.4049/jimmunol.173.1.21. [DOI] [PubMed] [Google Scholar]
- 8.Chen JF, Sonsalla PK, Pedata F, Melani A, Domenici MR, Popoli P, Geiger J, Lopes LV, de MA. Adenosine A2A receptors and brain injury: broad spectrum of neuroprotection, multifaceted actions and "fine tuning" modulation. Prog Neurobiol. 2007;83:310–331. doi: 10.1016/j.pneurobio.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Schwarzschild MA, Agnati L, Fuxe K, Chen JF, Morelli M. Targeting adenosine A2A receptors in Parkinson's disease. Trends Neurosci. 2006;29:647–654. doi: 10.1016/j.tins.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS, Schwarzschild MA. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huo Y, Zhao L, Hyman MC, Shashkin P, Harry BL, Burcin T, Forlow SB, Stark MA, Smith DF, Clarke S, Srinivasan S, Hedrick CC, Pratico D, Witztum JL, Nadler JL, Funk CD, Ley K. Critical role of macrophage 12/15-lipoxygenase for atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;110:2024–2031. doi: 10.1161/01.CIR.0000143628.37680.F6. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Tang R, Zhang W, et al. Core2 1-6-N-Glucosaminyltransferase-I Is Crucial for the Formation of Atherosclerotic Lesions in Apolipoprotein E-Deficient Mice. Arterioscler Thromb Vasc Biol. 2009;29:180–187. doi: 10.1161/ATVBAHA.108.170969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 14.Arai S, Shelton JM, Chen M, Bradley MN, Castrillo A, Bookout AL, Mak PA, Edwards PA, Mangelsdorf DJ, Tontonoz P, Miyazaki T. A role for the apoptosis inhibitory factor AIM/Spalpha/Api6 in atherosclerosis development. Cell Metab. 2005;1:201–213. doi: 10.1016/j.cmet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Liu B, Wang P, Dong X, Fernandez-Hernando C, Li Z, Hla T, Li Z, Claffey K, Smith JD, Wu D. Phospholipase C beta3 deficiency leads to macrophage hypersensitivity to apoptotic induction and reduction of atherosclerosis in mice. J Clin Invest. 2008;118:195–204. doi: 10.1172/JCI33139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li AC, Brown KK, Silvestre MJ, Willson TM, Palinski W, Glass CK. Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2000;106:523–531. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Bui TN, Xiang J, Lin A. Cyclic AMP inhibits p38 activation via CREB-induced dynein light chain. Mol Cell Biol. 2006;26:1223–1234. doi: 10.1128/MCB.26.4.1223-1234.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillis JW. The effects of selective A1 and A2a adenosine receptor antagonists on cerebral ischemic injury in the gerbil. Brain Res. 1995;705:79–84. doi: 10.1016/0006-8993(95)01153-6. [DOI] [PubMed] [Google Scholar]
- 19.Chen JF, Xu K, Petzer JP, Staal R, Xu YH, Beilstein M, Sonsalla PK, Castagnoli K, Castagnoli N, Jr, Schwarzschild MA. Neuroprotection by caffeine and A(2A) adenosine receptor inactivation in a model of Parkinson's disease. J Neurosci. 2001;21:RC143. doi: 10.1523/JNEUROSCI.21-10-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fink JS, Kalda A, Ryu H, Stack EC, Schwarzschild MA, Chen JF, Ferrante RJ. Genetic and pharmacological inactivation of the adenosine A2A receptor attenuates 3-nitropropionic acid-induced striatal damage. J Neurochem. 2004;88:538–544. doi: 10.1046/j.1471-4159.2003.02145.x. [DOI] [PubMed] [Google Scholar]
- 21.Marcoli M, Raiteri L, Bonfanti A, Monopoli A, Ongini E, Raiteri M, Maura G. Sensitivity to selective adenosine A1 and A2A receptor antagonists of the release of glutamate induced by ischemia in rat cerebrocortical slices. Neuropharmacology. 2003;45:201–210. doi: 10.1016/s0028-3908(03)00156-4. [DOI] [PubMed] [Google Scholar]
- 22.Yu L, Huang Z, Mariani J, Wang Y, Moskowitz M, Chen JF. Selective inactivation or reconstitution of adenosine A2A receptors in bone marrow cells reveals their significant contribution to the development of ischemic brain injury. Nat Med. 2004;10:1081–1087. doi: 10.1038/nm1103. [DOI] [PubMed] [Google Scholar]
- 23.Day YJ, Huang L, McDuffie MJ, Rosin DL, Ye H, Chen JF, Schwarzschild MA, Fink JS, Linden J, Okusa MD. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest. 2003;112:883–891. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohsenin A, Mi T, Xia Y, Kellems RE, Chen JF, Blackburn MR. Genetic removal of the A2A adenosine receptor enhances pulmonary inflammation, mucin production, and angiogenesis in adenosine deaminase-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L753–L761. doi: 10.1152/ajplung.00187.2007. [DOI] [PubMed] [Google Scholar]
- 25.Pinhal-Enfield G, Ramanathan M, Hasko G, Vogel SN, Salzman AL, Boons GJ, Leibovich SJ. An angiogenic switch in macrophages involving synergy between Toll-like receptors 2, 4, 7, and 9 and adenosine A(2A) receptors. Am J Pathol. 2003;163:711–721. doi: 10.1016/S0002-9440(10)63698-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller YI, Chang MK, Binder CJ, Shaw PX, Witztum JL. Oxidized low density lipoprotein and innate immune receptors. Curr Opin Lipidol. 2003 October;14(5):437–445. doi: 10.1097/00041433-200310000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Kanters E, Pasparakis M, Gijbels MJ, Vergouwe MN, Partouns-Hendriks I, Fijneman RJ, Clausen BE, Forster I, Kockx MM, Rajewsky K, Kraal G, Hofker MH, de Winther MP. Inhibition of NF-kappaB activation in macrophages increases atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;112:1176–1185. doi: 10.1172/JCI18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kockx MM. Apoptosis in the atherosclerotic plaque: quantitative and qualitative aspects. Arterioscler Thromb Vasc Biol. 1998;18:1519–1522. doi: 10.1161/01.atv.18.10.1519. [DOI] [PubMed] [Google Scholar]
- 29.Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1256–1261. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- 30.Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- 31.Noguchi T, Ishii K, Fukutomi H, Naguro I, Matsuzawa A, Takeda K, Ichijo H. Requirement of reactive oxygen species-dependent activation of ASK1-p38 MAPK pathway for extracellular ATP-induced apoptosis in macrophage. J Biol Chem. 2008;283:7657–7665. doi: 10.1074/jbc.M708402200. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan GW, Fang G, Linden J, Scheld WM. A2A adenosine receptor activation improves survival in mouse models of endotoxemia and sepsis. J Infect Dis. 2004;189:1897–1904. doi: 10.1086/386311. [DOI] [PubMed] [Google Scholar]
- 33.Reutershan J, Cagnina RE, Chang D, Linden J, Ley K. Therapeutic anti-inflammatory effects of myeloid cell adenosine receptor A2a stimulation in lipopolysaccharide-induced lung injury. J Immunol. 2007;179:1254–1263. doi: 10.4049/jimmunol.179.2.1254. [DOI] [PubMed] [Google Scholar]
- 34.Awad AS, Huang L, Ye H, Duong ET, Bolton WK, Linden J, Okusa MD. Adenosine A2A receptor activation attenuates inflammation and injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2006;290:F828–F837. doi: 10.1152/ajprenal.00310.2005. [DOI] [PubMed] [Google Scholar]
- 35.Odashima M, Bamias G, Rivera-Nieves J, Linden J, Nast CC, Moskaluk CA, Marini M, Sugawara K, Kozaiwa K, Otaka M, Watanabe S, Cominelli F. Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology. 2005;129:26–33. doi: 10.1053/j.gastro.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 36.Reiss AB, Rahman MM, Chan ES, Montesinos MC, Awadallah NW, Cronstein BN. Adenosine A2A receptor occupancy stimulates expression of proteins involved in reverse cholesterol transport and inhibits foam cell formation in macrophages. J Leukoc Biol. 2004;76:727–734. doi: 10.1189/jlb.0204107. [DOI] [PubMed] [Google Scholar]
- 37.McPherson JA, Barringhaus KG, Bishop GG, Sanders JM, Rieger JM, Hesselbacher SE, Gimple LW, Powers ER, Macdonald T, Sullivan G, Linden J, Sarembock IJ. Adenosine A(2A) receptor stimulation reduces inflammation and neointimal growth in a murine carotid ligation model. Arterioscler Thromb Vasc Biol. 2001;21:791–796. doi: 10.1161/01.atv.21.5.791. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.