Abstract
Goal
This study aimed to determine the frequency of frontotemporal lobar degeneration with ubiquitinated inclusions (FTLD-U) in the setting of hippocampal sclerosis (HpScl) and Alzheimer's disease (AD) using immunohistochemistry for TAR DNA binding protein 43 (TDP-43), a putative marker for FTLD-U.
Methods
Initially, 21 cases of HpScl associated with a variety of other pathologic processes and 74 cases of AD were screened for FTLD-U with TDP-43 immunohistochemistry. A confirmation study was performed on 93 additional AD cases. Specificity of TDP-43 antibodies was assessed using double immunolabeling confocal microscopy, immunoelectron microscopy and biochemistry.
Results
TDP-43 immunoreactivity was detected in 71% of HpScl and 23% of AD cases. Double immunostaining of AD cases for TDP-43 and phospho-tau showed that the TDP-43 immunoreactive inclusions were usually distinct from neurofibrillary tangles. At the ultrastructural level TDP-43 immunoreactivity in AD was associated with granular and filamentous cytosolic material and only occasionally associated with tau filaments. Western blots of AD cases revealed a band that migrated at a higher molecular weight than normal TDP-43 that was not present in AD cases without TDP-43 immunoreactivity.
Interpretation
The present results suggest that as many as 20% of AD cases and more than 70% of HpScl cases have pathology similar to that found in FTLD-U. Whether this represents concomitant FTLD-U or is analogous to colocalization of α-synuclein and tau in AD, reflecting a propensity for co-deposition of abnormal protein conformers, remains to be determined.
Keywords: Alzheimer's disease, biochemistry, electron microscopy, frontotemporal lobar degeneration with ubiquitinated inclusions (FTLD-U), hippocampal sclerosis, immunohistochemistry, TAR DNA binding protein 43 (TDP-43)
Introduction
Frontotemporal lobar degeneration (FTLD) is a heterogeneous group of neurodegenerative disorders that are subdivided into tau-positive and tau-negative disorders according to whether or not there are tau immunoreactive neuronal and glial lesions and abnormal conformers of tau protein on biochemical analyses.1 FTLD with ubiquitin immunoreactive neuronal inclusions (FTLD-U) is most common.2-5 The hallmarks of FTLD-U are neuronal cytoplasmic and nuclear inclusions and neurites that are positive for ubiquitin and negative for both tau and α-synuclein.6-8
The association of FTLD-U with other neurodegenerative disorders has not been amply studied, in part, because no specific marker for FTLD-U exists. Since inclusions in many disorders and in aging show ubiquitin immunoreactivity, it has been difficult to distinguish inclusions related to FTLD-U from inclusions due to other pathologic processes or age-related changes. One of the few studies that addressed mixed pathology found that approximately 16% of patients with FTLD-U have concomitant Alzheimer's disease (AD).9 In another study Alzheimer pathology was detected in 30% of patients with amyotrophic lateral sclerosis (ALS) dementia, which is usually due to FTLD-U.10 The converse, namely the occurrence of FTLD-U in AD, has not been studied.
Neumann and coworkers reported TAR DNA binding protein 43 (TDP-43) in neuronal inclusions and neurites of FTLD-U and in ALS,11 and suggested that TDP-43 may be a specific marker for FTLD-U and ALS. A recent report suggests that this may not be entirely true, since neuronal inclusions in Pick's disease may sometimes be immunoreactive for TDP-43.12 Despite this caveat, the discovery of TDP-43 immunoreactivity in FTLD-U inclusions offers promise that, like discovery of α-synuclein, immunohistochemistry for TDP-43 may permit detection of FTLD-U in the setting of concurrent AD, analogous to immunohistochemistry for α-synuclein for detection of Lewy bodies in the setting of concurrent AD.13
Hippocampal sclerosis (HpScl) is a pathologic finding characterized by neuronal loss in the subiculum and CA1 region of the hippocampus. HpScl is associated with a variety of disorders. When HpScl occurs in the setting of AD, it is often difficult to distinguish from neuronal loss due to neurofibrillary tangles (NFTs). In such cases there are usually numerous extracellular NFTs. Only when neuronal loss is disproportionate to the density of extracellular NFTs is a diagnosis of HpScl warranted, and there are some investigators that do not recognize HpScl in the setting of AD.14 In contrast, HpScl is a widely recognized feature of FTLD-U, being found in approximately 70% of cases.15, 16 Up to 12% of HpScl cases have been found to have FTLD-U.17 Due to this strong association, when HpScl is detected, it is important to rule out FTLD-U.
The goal of this study was to use TDP-43 immunohistochemistry to determine the frequency of FTLD-U in AD with and without HpScl, as well as in HpScl associated with a variety of other pathologic processes. The specificity of TDP-43 was assessed with double immunolabeling confocal microscopy, immunoelectron microscopy and biochemistry.
Materials and Methods
Case material
Initial series
The brain bank at Mayo Clinic in Jacksonville database from 1998 to June 2006 was searched for cases with HpScl and adequate material for additional histologic studies. There were 108 cases of HpScl, including 43 cases of FTLD-U, 44 cases of AD and 10 cases of HpScl with a variety of other neurodegenerative disorders. There were also 4 cases in which HpScl was associated with extensive cerebrovascular disease and considered to be anoxicischemic in nature and 7 cases in which HpScl was deemed to be the major pathologic process (including 3 patients with epilepsy). For the purposes of this study the latter two groups were combined as “pure HpScl” (Table 1).
Table 1.
Demographics and Pathology of All Cases
| Initial series |
Confirmation Series | ||||
|---|---|---|---|---|---|
| HpScl/pure (n=11) | HpScl/other (n=10) | HpScl/AD (n=44) | AD (n=30) | AD (n=93) | |
| Age at death, mean ± SD (y) | 80 ± 17 | 78 ± 8 | 85 ± 6 | 85± 5 | 79 ± 9 |
| Sex, M:F | 7:4 | 6:4 | 22:22 | 9:21 | 46:47 |
| Braak stage, median (25%-tile, 75%-tile) | 2 (0.25, 2.9) | 2 (1, 2.5) | 5 (4.5, 6) | 5.8 (5, 6) | 5.5 (5, 6) |
| Brain weight, mean ± SD (g) | 1170 ± 220 | 1120 ± 200 | 1020 ± 150 | 1000 ± 110 | 1040 ± 160 |
| TDP-43 immunoreactive lesions, number of cases (%) |
8 (73%) |
5 (50%) |
33 (75%) |
9 (30%) |
19 (20%) |
| Other pathology (number of cases) | |||||
| Age-associated senile plaques | 3 | 1 | |||
| Cerebrovascular disease | 4 | 1 | 16 | 14 | 22 |
| Argyrophilic grains | 3 | 6 | 2 | 0 | 6 |
| Lewy body disease | 5 | 14 | 11 | 20 | |
| Corticobasal degeneration | 2 | ||||
| Progressive supranuclear palsy | 1 | ||||
| Multiple system atrophy | 1 | ||||
| Huntington's disease | 1 | ||||
| Intranuclear hyaline inclusion disease | 1 | ||||
All cases had previously undergone a neuropathological assessment that included evaluation of gross and microscopic findings, as well as quantitative analysis of Alzheimer type pathology. A Braak NFT stage was assigned to all cases based upon the distribution of NFTs with thioflavin-S fluorescent microscopy, as previously described.13, 18 Most cases underwent immunostaining with for phospho-tau (CP1319), α-synuclein and ubiquitin.
The 65 HpScl cases were not originally thought to be related to FTLD-U. The pure HpScl cases (HpScl/pure) had no histopathologic features of neurodegenerative disorders other than age-associated changes, including sparse senile plaques, isolated cerebrovascular lesions or medial temporal lobe argyrophilic grains. The second group (HpScl/other) had HpScl associated with other neurodegenerative disorders, such as Lewy body disease or corticobasal degeneration (Table 1). Some of these cases also had senile plaques, isolated cerebrovascular lesions or argyrophilic grains. The third group (HpScl/AD) had numerous senile plaques and a Braak NFT ≥IV and sufficient to warrant a diagnosis of high likelihood AD using current neuropathologic criteria.
In addition, 30 AD cases without HpScl were selected to match HpScl/AD cases with respect to Braak NFT stage, age, brain weight, source of tissue and age at death (Table 1). Other controls included cases with no significant pathology (n=20) between ages 20 and 90 years and FTLD-U with HpScl (n=10).
Confirmation series
An independent series of 93 AD cases (Table 1) was subsequently screened for TDP-43 to validate the findings from the initial series. These AD cases were State of Florida Alzheimer Disease Initiative cases 17 from one geographic region, Miami Beach, and all lacked HpScl. They differed from the initial series of AD cases only in that they were younger.
TDP-43 immunohistochemistry
Five-micron thick sections of formalin-fixed, paraffin-embedded tissue from medial temporal lobe were immunostained with a DAKO-Autostainer (DAKO-Cytomaton, Carpinteria, CA) for TDP-43 (rabbit polyclonal antibody; 1:3,000; ProteinTech Group, Inc., Chicago, IL). In a subset of cases immunostaining was also performed with a mouse monoclonal antibody (1:2000; Abnova Corp., Taipei, Taiwan), which gave identical results (Fig 1). For the purpose of analysis, only the data for rabbit antiserum are reported. Immunohistochemical procedures were similar to previous reports.13 The primary antibodies used in this study and their dilutions are summarized in Table 2.
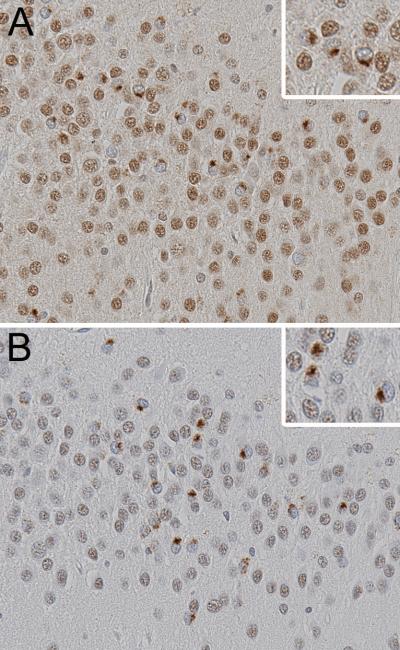
Fig 1.
Comparison of TDP-43 antibodies, (A) mouse monoclonal and (B) rabbit polyclonal, with immunohistochemistry of adjacent sections of the same case reveals neuronal cytoplasmic inclusions (inset at higher magnification) in a subset of neurons in the hippocampal dentate fascia. Note also the staining of normal nuclei and the absence of nuclear staining in neurons with cytoplasmic inclusions. Images are ×400 and inset ×800.
Table 2.
Antibody Specifications
| Antibody | Type | Epitope | Source | Dilution |
|---|---|---|---|---|
| TDP-43 | Polyclonal affinity purified Ig | TDP-43 | ProteinTech, Chicago, IL | 1:3,000 (IHC) 1:2,000 (IF) 1:10 (IEM) |
| TDP-43 | Monoclonal IgG1 | TDP-43 | Abnova, Taipei, Taiwan | 1:2,000 (IHC) |
| CP13 | Monoclonal IgG1 | Phospho-tau | Jicha, et al.19 | 1:200 (IF) |
| PHF-1 | Monoclonal IgG1 | Phospho-tau | Greenberg, et al.20 | 1:500 (IF) Neat (IEM) |
IHC, Immunohistochemistry; IF, Immunofluorescence; IEM – immunoelectron microscopy
Semiquantitative histopathologic analyses
The presence and severity of TDP-43 immunoreactivity was assessed in hippocampus, parahippocampal gyrus, occipitotemporal gyrus and inferior temporal gyrus. The presence of TDP-43 immunoreactivity in neuronal cytoplasmic and intranuclear inclusions, as well as curvilinear, ellipsoid and dot-like neurites was noted. For cases with any TDP-43 immunoreactivity in the hippocampus or adjacent cortices, additional sections of basal forebrain, midfrontal cortex, caudate nucleus and cingulate gyrus were also studied with TDP-43 immunohistochemistry. TDP-43 immunoreactivity was scored in the amygdala, nucleus accumbens, middle frontal gyrus, cingulate gyrus, dentate fascia and inferior temporal cortex. A three point grading scale (0 - none; 0.5 - mild to moderate; and 1 - severe) was used to evaluate the severity of TDP-43 immunoreactivity. No attempt was made to score cytoplasmic and neuritic lesions separately, but most cases with a high TDP-43 score had dystrophic neurites in addition to neuronal cytoplasmic inclusions.
TDP-43 and phospho-tau confocal microscopy
Specificity of TDP-43 immunohistochemistry was assessed with double labeling confocal microscopy for TDP-43 and phospho-tau, CP13, which detects pretangles and intracellular NFTs). In a subset of cases an identical procedure was used with PHF-1,20 which unlike CP13 detects both extracellular and intracellular NFTs. After removing paraffin, the sections were treated with 5% normal goat serum and incubated for 90 minutes in a cocktail of TDP-43 and CP13 or PHF-1. Sections were subsequently rinsed with PBS and incubated for an additional 90 minutes at room temperature in a cocktail of secondary antibodies: goat anti-rabbit Alexa Flour 488 and goat anti-mouse Alexa Flour 568 (1:250, Molecular Probes-Invitrogen, Eugene, OR). Sections were washed and coverslipped with Vectashield (Vector Laboratories, Burlingame, CA) and observed with a confocal microscope (Fluoview Version 2.0, Olympus, Melville, NY) with an Olympus BX50 microscope. For quantitative analysis, three images were captured from both the dentate fascia and the entorhinal cortex under a 40X objective. The number of neurons with single and double labeling for tau and TDP-43 was determined by manual counts from the digital images.
Electron microscopy
Small samples were dissected from the entorhinal cortex of formalin-fixed brains, including a case of FTLD-U as a positive control and two cases of AD with TDP-43 immunoreactive pathology. An AD case that had no TDP-43 immunoreactive pathology was the negative control, as was a sample from a transgenic mouse (Tg4510) that had abundant NFTs.21 All samples were processed identically and embedded in LR White embedding medium as previously described.22 Nickel grids containing thin sections were floated with the section-side down on 2 ml of citrate buffer, pH 6, in a 100° C oven for 10 minutes, and cooled to room temperature for 15 minutes prior to immunogold electron microscopy.22 Rabbit antibody to TDP-43 was diluted 1:10 in PBS, pH 7. Some sections were double stained with rabbit TDP-43 and mouse monoclonal antibody to phospho-tau (PHF-120, undiluted supernatant) using gold particles of two different sizes (5-nm and 18-nm) conjugated to species-specific secondary antibodies.
TDP-43 biochemistry
Biochemical analyses were performed to characterize TDP-43 in 6 cases of AD that had TDP-43 immunoreactive neuronal inclusions, 3 cases of AD that did not have TDP-43 immunoreactivity, 1 case of HpScl with TDP-43 immunoreactive neuronal inclusions, 4 cases of FTLD-U and 2 elderly normals with no significant pathology. Samples of frozen hippocampus, superior temporal gyrus or middle frontal gyrus were stripped of meninges and dissected from white matter. The gray matter was homogenized and subjected to sequential fractionation as described by Neumann, et al.11 Low salt, high salt, sarkosyl and urea fractions were obtained and aliquots were run on 15% Tris-HCl SDS-polyacrylamide gels. The amount of sample loaded per lane was normalized to the signal intensity of glyceraldehyde phosphate dehydrogenase. Proteins were transferred to polyvinylidene difluoride membranes, probed with rabbit antibody to TDP-43, followed by anti-rabbit antibody conjugated to Alexa 680 (1:1000; Molecular Probes, Carlsbad, CA). Blots were imaged using the Odyssey Infrared Imager (Licor BioSciences, Lincoln, NB).
To assess the phosphatase sensitivity of TDP-43, samples were treated with phosphatase. A 50 μl aliquot from the urea fraction of cases of AD with and without TDP-43 immunoreactivity, FTLD-U, HpScl with TDP-43 immunoreactivity and normal controls were dialyzed for 40 minutes against 50 mM Tris-HCl, pH 7.5. Dialysis was performed on the surface of a 25 mm VS filter floating on 5 ml of Tris buffer. Dephosphorylation of the dialyzed samples was performed with 2 U of shrimp alkaline phosphatase for 30 min at 37°C. Samples were then processed as above for immunoblots.
Statistical analyses
Unpaired Student's t-tests were used to analyze differences between groups for age and brain weight, while non-parametric variables, such as Braak stage and TDP-43 score, were analyzed with Mann-Whitney U test. Chi-square and Fisher's Exact Tests were used to analyze differences in dichotomous variables, such as sex and presence of NII or other diseases. Relations between demongraphic and pathologic variable were assessed with Spearman Rank Order Correlations. Statistical analyses were performed using Sigma Stat for Windows, version 3.11 (Systat Software, Richmond, CA) and a significance level was set at p<0.05.
RESULTS
TDP-43 immunohistochemistry in HpScl
None of the cases of HpScl was considered to have FTLD-U based upon initial gross and microscopic studies. In some cases HpScl was associated with cerebrovascular pathology and was attributed to hypoxic-ischemic injury. In other cases there was no clear etiology. Three cases had HpScl associated with epilepsy (none had TDP-43 immunoreactivity). Nine cases had HpScl associated with tauopathy (argyrophilic grain disease, corticobasal degeneration or progressive supranuclear palsy) and 2 of these cases were similar to what has been described as “hippocampal sclerosis dementia tauopathy” (both were positive for TDP-43).23 The MSA case (which was TDP-43 positive) had a history of severe orthostatic hypotension, and HpScl was initially attributed to cerebral hypoperfusion injury. It is the only case of MSA in the neuropathology files (N=50) with HpScl.
Immunoreactivity for TDP-43 was detected in neuronal cytoplasmic inclusions and neurites of 13 cases of HpScl/pure and HpScl/other. The lesions were morphologically similar to those in FTLD-U11, 24, 25 (Fig 2). Several different schemes have been proposed to classify TDP-43 immunoreactivity in FTLD-U,24-26 but there is no consensus on their validity. Nevertheless, 38% of the cases had abundant cortical neurites in upper cortical layers similar to Type 1 of Mackenzie, et al.24 and Type 3 of Sampathu, et al.25
Fig 2.
AD case with HpScl showing TDP-43 positive cytoplasmic inclusions and neurites in the dentate fascia (A), entorhinal cortex (B), nucleus accumbens (C), cingulate gyrus (D) and amygdala (E). Intranuclear neuronal inclusions are detected in the entorhinal cortex in some cases (F). Images are ×400 and inset ×800.
TDP-43 immunoreactivity was detected in 8 cases of HpScl/pure and 5 cases of HpScl/other. The demographic and pathologic features of HpScl cases that had TDP-43 immunoreactivity are summarized in Table 3. All 20 normal controls, ranging from 20 to 90 years of age, were negative for TDP-43 immunoreactivity, while all 10 cases of clinically and pathologically typical FTLD-U were positive. (The FTLD-U and normal control cases are not included in the Tables 1 and 3.)
Table 3.
Demographics and Pathology of Cases with TDP-43 Immunoreactivity
| Initial series |
Confirmation series | ||||
|---|---|---|---|---|---|
| HpScl/pure (n=8) | HpScl/other (n=5) | HpScl/AD (n=33) | AD (n=9) | AD (n=19) | |
| Age at death, mean ± SD (y) | 87 ± 4 | 84 ± 6 | 86 ± 6 | 84 ± 4 | 82 ± 7 |
| Sex, M:F | 5:3 | 3:2 | 17:16 | 3:6 | 9:10 |
| Braak stage, median (25%-tile, 75%-tile) | 2.2 (1.5, 2.8) | 2.5 (2, 3) | 5 (4.5, 6) | 6 (5, 6) | 5.5 (5, 6) |
| Brain weight, mean ± SD (g) |
1130 ± 220 |
1110 ± 82 |
1020 ± 140 |
1040 ± 74 |
990 ± 130 |
| Other pathology (number of cases) | |||||
| Hippocampal sclerosis | 8 | 5 | 33 | 0 | 0 |
| Alzheimer's disease | 0 | 0 | 33 | 9 | 19 |
| Cerebrovascular disease | 4 | 0 | 12 | 2 | 7 |
| Argyrophilic grains | 2 | 3 | 1 | 0 | 0 |
| Lewy bodies | 0 | 4 | 9 | 4 | 5 |
| Multiple system atrophy | 0 | 1 | 0 | 0 | 0 |
Distribution of TDP-43 immunoreactivity
The density and distribution of TDP-43 immunoreactivity are summarized in Table 4. All cases had some neuronal inclusions in the dentate fascia of the hippocampus and neuronal inclusions and neurites in the entorhinal cortex. Extension of the pathology beyond the limbic lobe was not uniform. Some cases had involvement of the occipitotemporal and inferior temporal gyri. These cases also had more severe pathology outside of the temporal lobe and were considered to have “diffuse” TDP-43 immunoreactivity. Cases with involvement relatively confined to the limbic lobe were referred to as “limbic” (Fig 3), using terminology along the lines used to classify Lewy body disease.27 The lesions in diffuse and limbic types were morphologically similar, but NII tended to be more frequent in diffuse types.
Table 4.
Distribution of TDP-43 Immunoreactivity
| Initial series |
Confirmation series | ||||
|---|---|---|---|---|---|
| HpScl/pure (n=8) | HpScl/other (n=5) | HpScl/AD (n=33) | AD (n=9) | AD (n=19) | |
| TDP-43 immunoreactivity score (median, 25%-tile, 75%-tile) and percent affected | |||||
| Dentate fascia | 1 (1, 1) 100% |
0.5 (0.5, 1) 100% |
1 (1, 1) 100% |
1 (0.5, 1) 100% |
1 (0.5, 1) 100% |
| Entorhinal cortex | 1 (1, 1) 100% |
1 (0.9, 1) 100% |
1 (1, 1) 100% |
1 (0.9, 1) 100% |
1 (0.6, 1) 100% |
| Occipitotemporal cortex | 0.5 (0.2, 0.5) 75% |
0.5 (0, 0.5) 60% |
0.5 (0.5, 1) 94% |
0.5 (0, 0.5) 56% |
0.5 (0, 0.5) 63% |
| Inferior temporal cortex | 0 (0, 0) 13% |
0 (0, 0) 0% |
0 (0, 0.5) 44% |
0 (0, 0) 11% |
0 (0, 0) 21% |
| Cingulate gyrus | nd | nd | 0.5 (0, 1) 64% |
0 (0, 0.4) 29% |
nd |
| Amygdala | nd | nd | 1 (1, 1) 100% |
1 (1, 1) 100% |
nd |
| Nucleus accumbens | nd | nd | 0.5 (0.5, 1) 79% |
0.5 (0, 0.6) 60% |
nd |
| Midfrontal gyrus | nd | nd | 0 (0, 0) 13% |
0 (0, 0) 0% |
nd |
| Number of cases with NII | 2 | 0 | 5 | 1 | 1 |
| TDP-43 type (diffuse:limbic) | 0:8 | 0:5 | 13:20 | 1:8 | 4:15 |
nd = not done
Fig 3.

Distribution of TDP-43 immunoreactive inclusions. (A) The diffuse type has inclusions in the dentate fascia of the hippocampus (hp), the entorhinal cortex (erc), the occipitotemporal gyrus (otg) as well as the inferior temporal gyrus (itg). (B) The limbic type showed immunoreactivity limited to the dentate fascia and entorhinal cortex, with sparse or no involvement of the occipitotemporal gyrus.
TDP-43 in AD
In addition to the 44 cases of AD with HpScl, 30 cases of AD without HpScl were also screened. In HpScl/AD 75% (33/44) were positive, while in AD without HpScl 30% (9/30) were positive. As in cases with HpScl, when TDP-43 immunoreactivity was detected in AD, it was present in the dentate fascia and the entorhinal cortex, but variably present outside the limbic structures. Almost half the cases (49%) had abundant neurites. Analysis of other brain regions showed consistent involvement of the amygdala, with less frequent involvement in other limbic structures such as the cingulate gyrus and the nucleus accumbens. It was often not detected in the convexity gray matter of the midfrontal gyrus (Table 4).
The diffuse type of TDP-43 immunoreactivity was found in 14 (33%) AD cases (13 with HpScl and 1 without HpScl), while the limbic type was more common, being found in 28 cases (67%) (20 with HpScl and 8 without HpScl). Cases of the diffuse type had significantly more TDP-43 immunoreactivity in cingulate gyrus and nucleus accumbens than the limbic type (median cingulate score: 1 versus 0, p<0.01; median nucleus accumbens score: 1 versus 0.5, p< 0.05). In addition, NII were more frequent in the diffuse type (4% versus 36%, p=0.01).
There were no differences in age, sex, Braak stage, brain weight or frequency of other pathologies for AD cases with TDP-43 immunoreactive lesions with respect to whether or not they had HpScl. On the other hand, cases of AD with HpScl tended to have more severe pathology in the occipitotemporal gyrus compared to AD cases that did not have HpScl (p=0.02).
Frequency of TDP-43 immunoreactivity in AD: confirmation series
To confirm the presence of TDP-43 immunoreactivity in AD, an independent series of 93 AD cases was studied (Tables 1 and 3). TDP-43 immunoreactive neuronal inclusions and neurites were detected in 19 cases (20%). The morphology of the lesions was similar to lesions in FTLD-U, and 32% had abundant neuritic pathology The frequency of TDP-43 immunoreactive inclusions in the confirmation series was not significantly different from the initial series of AD cases without HpScl (Chi-square = 0.700, 1 df, p=0.40). The distribution of TDP-43 immunoreactivity was similar to the initial AD series without HpScl; most cases were limbic type (Table 4).
In addition to FTLD-U like pathology, a small number of NFTs in the subiculum and entorhinal cortex were noted to have TDP-43 immunoreactivity in this larger series of AD cases (Fig 4). In these cases the only TDP-43 immunoreactivity was in NFT and not in neurites. Of the 74 cases without FTLD-U type pathology, NFT-like TDP-43 immunoreactivity was detected in 10 (14%). When all 123 AD cases without HpScl were combined (30 original and 93 validating series), there was a correlation between Braak NFT stage and the frequency of NFT-like TDP-43 immunoreactivity (Spearman rank order correlation r=0.24, p=0.01). In contrast, there was no correlation between Braak NFT stage and FTLD-U type TDP-43 immunoreactivity (Spearman rank order correlation r=0.03, p=0.73).
Fig 4.

In AD cases that did not have FTLD-U-like TDP-43 immunoreactivity, occasional NFTs in the subiculum (and less often the entorhinal cortex) have TDP-43 immunoreactivity. An adjacent extracellular NFT (arrow) shows no staining. Inset shows a confocal microscopic image of a NFT double stained for phosphotau (green) and TDP-43 (red) showing only partial overlap of phospho-tau and TDP-43 immunoreactivities in NFTs. Images are ×400
Double labeling confocal microscopy of phospho-tau (CP13) and TDP-43
Double labeling confocal microscopy showed co-localization of phospho-tau and TDP-43 that varied by anatomic region, with more co-localization in the entorhinal cortex than in the dentate fascia. There were also two different patterns of colocalization of TDP-43 and phosphotau. In one there was double labeling of the inclusion with overlap of the two epitopes. In the other the two epitopes were intermingled, but separate structures (Fig 5). In some neurons NFT were positive for phospho-tau, while a separate round or curvilinear inclusion within the same neuron was positive for TDP-43. Separate inclusions within the same neuron were more common than co-localization of phospho-tau and TDP-43 within the same inclusion (Table 5). The results with PHF-1 were similar to those with CP13 (not shown).
Fig 5.
Double-labeling for phospho-tau (A and D) (green) and TDP-43 (B and E) (red) with merged images (C and F) shows two types of co-localization of phospho-tau and TDP-43. In Type 1 (D, E and F) there is overlap of the epitopes in some of the inclusions, while other inclusions are single labeled for either tau or TDP-43. In Type 2 (A, B and C) there is intermingling, but separation, of the two epitopes within the same neuron, as well as inclusions that are single labeled for either tau or TDP-43. All images are ×400.
Table 5.
TDP-43 and Phospho-Tau Double Labeled Neurons in AD
| Overlapping epitopes in same inclusion | Separate epitopes in the same neuron | All | |
|---|---|---|---|
| Dentate fascia (n=118) | 3 (3%) | 17 (14%) | 20 (17%) |
| Entorhinal cortex (n=160) | 30 (19%)# | 68 (43%)* | 98 (61%)‡ |
| Total (n=278) | 33 (12%) | 85 (31%) | 118 (42%)† |
p<0.05 (dentate fascia vs. entorhinal)
p<0.01 (dentate fascia vs. entorhinal)
p<0.001 (dentate fascia vs. entorhinal for All)
p<0.01 (overlapping vs separate epitopes)
TDP-43 immunoelectron microscopy in AD
Immunoelectron microscopy for TDP-43 was performed in AD cases with and without TDP-43 immunoreactivity, as well as FTLD-U as a positive control. In FTLD-U cases TDP-43 was detected in granular and filamentous cytoplasmic inclusions as well as neuritic processes. The filaments were straight and had a diameter than ranged from 15-nm to 20-nm similar to previous reports using ubiquitin immunoelectron microscopy.28, 29 In AD cases with TDP-43 immunoreactive pathology, TDP-43 was present in granulofilamentous lesions in both cytoplasm and intranuclear inclusions and that were distinct from paired helical or straight tau filaments of NFTs (Fig 6). In a few neurons TDP-43 immunoreactivity was associated with tightly bundled paired helical filaments or straight tau filaments (confirmed with tau double labeling), although it was not possible to determine if this was binding to granular material associated with the filaments or binding directly to the filaments. In the same cell where tau filaments were dispersed and lacking granular material, there was no TDP-43 immunolabeling (Fig 6E). Arguing against TDP-43 immunoreactivity with tau is the fact that there was almost no TDP-43 immunolabeling of NFTs in AD cases without TDP-43 immunoreactivity at the light microscopic level and no labeling of NFTs in Tg4510 transgenic mice with immunoelectron microscopy.
Fig 6.
Immunoelectron microscopy of TDP-43 shows a neuron (A) with an intranuclear inclusion (arrow), nucleolus (No) and cytoplasmic filaments part of a NFT (double arrows). L, lipofuscin. Bar, 1 μm. In (B), an enlargement of the boxed area in (A) shows that the intranuclear inclusion composed of granule coated filaments of 15−20-nm in diameter that are heavily labeled with TDP-43 (gold particles in circles). Chromatin at the nuclear membrane is unlabeled (arrow). Bar, 0.1 μm. In (C) enlargement of the NFT reveals paired helical filaments (arrows) and straight filaments (curved arrows) that are unlabeled for TDP-43. The nucleolus is also unlabeled (not shown). Bar, 0.15 μm. In (D) a neuronal with NFT has tau filaments in tightly packed bundles (arrows) and randomly oriented in the cytoplasm (*). L, lipofuscin. Bar, 1 μm. In (E) the boxed area in (D) is enlarged to show that the bundled filaments tightly packed with dense granular material and heavy labeling for TDP-43 (gold particles in circles), while the randomly oriented straight tau filaments are mostly unlabeled. Bar, 0.3 μm.
TDP-43 biochemistry
Western blotting of urea extracts from normal brains and AD cases without TDP-43 inclusions for TDP-43 revealed a characteristic 43 kDa band11, 12 (Fig 7). In FTLD-U, AD with TDP-43 immunoreactive pathology with or without HpScl, and in a case of pure HpScl with TDP-43 immunoreactivity abnormal bands of approximately 45 kDa and a 25 kDa that have been described in FTLD-U were detected.11, 12. There was also TDP-43 immunoreactive material at the top of the stacking and running gels and variable high molecular weigh smearing in these cases. Phosphatase treatment of the urea extracts before immunoblotting produced a loss of immunoreactivity in the higher molecular weight species. There were also changes in the 25 kDa band (a singlet band became a doublet) (not shown).
Fig 7.
Western blot of urea fractions of hippocampal (H), temporal (T) and frontal cortices (F) from AD and HpScl TDP-43 immunoreactive cases (TDP-43+) exhibited abnormal bands of approximately 45 (black arrow) and 25 (white arrow) kDa, similar to what is seen in FTLD-U controls. This pattern was not found in AD and normal elderly individuals that did not have TDP-43 immunoreactive lesions (TDP-43−).
DISCUSSION
TDP-43 in HpScl
The present study demonstrates that a significant proportion (over 70%) of cases with HpScl in the elderly have TDP-43 immunoreactive neuronal inclusions and neurites that resemble those in FTLD-U. This is consistent with previous studies using ubiquitin immunohistochemistry to investigate the frequency of FTLD-U in HpScl,30 but the present series included cases of HpScl in the setting of AD or Lewy body disease, in which it heretofore has been difficult to detect FTLD-U because of ubiquitin in NFTs and Lewy bodies. The analogous situation existed for detecting Lewy bodies in the setting of AD prior to the discovery of α-synuclein as a marker for Lewy bodies. Only double staining for tau and ubiquitin provided a means to differentiate Lewy bodies (ubiquitin-positive, tau-negative) from NFTs (ubiquitin-positive, tau-positive).17 With the advent of α-synuclein immunohistochemistry, this simple dichotomy was also challenged when it was shown that a small proportion of Lewy bodies had tau immunoreactivity.31, 32 Nevertheless, for the most part, α-synuclein remains a sensitive and specific marker for Lewy body pathology even when it occurs in the setting of AD. The present results suggest that same may be true for TDP-43.
In AD extensive neuronal loss occurs in CA1 and the subiculum of the hippocampus due to neurofibrillary degeneration. This is the same distribution of neuronal loss in HpScl due to FTLD-U or hypoxic-ischemic injury. In AD a diagnosis of HpScl is considered when there is disproportionate neuronal loss to the number of NFTs. The presence of TDP-43 immunoreactive inclusions in 70% of cases suggests that TDP-43 immunohistochemistry may provide a means to diagnose FTLD-U in the setting of AD with HpScl.
TDP-43 in AD
The present study showed that TDP-43 immunoreactive lesions were present in over 20% of AD cases that did not have HpScl. Given the high frequency of FTLD-U in cases with HpScl,30 these results suggest that AD cases with HpScl and TDP-43 immunoreactive inclusions might have mixed dementia – AD/FTLD-U. Most of the TDP-43 immunoreactive structures were histologically similar to those found in FTLD-U (although a few were clearly coexistent with NFTs) based upon double immunohistochemistry for TDP-43 and phospho-tau and immunoelectron microscopy. Cytoplasmic localization of TDP-43 is pathologic since it is normally a nuclear DNA binding protein. When TDP-43 was detected in the cytoplasm, either alone or in association with phospho-tau, there was no nuclear labeling, similar to previous reports in FTLD-U.11 Biochemical studies in AD with TDP-43 immunoreactive pathology revealed abnormal conformers of TDP-43 similar to those found in FTLD-U.11 These high molecular weight phosphorylated TDP-43 conformers were not detected in AD without TDP-43 immunoreactivity or in normal controls, but they were present in FTLD-U.
Neuronal populations that were vulnerable to TDP-43 inclusions were in the limbic lobe. In this study, TDP-43 immunoreactivity was most frequent in hippocampus, entorhinal cortex, amygdala, cingulate gyrus and nucleus accumbens, but less often in temporal and frontal multi-modal association cortices. Analogous to Lewy body disorders, some cases had TDP-43 relatively confined to the limbic lobe (“limbic type”) while others had more widespread (“diffuse type”) neuronal inclusions. The present results suggest that this difference is related to severity of the process since limbic and diffuse types were similar morphologically and demographically.
TDP-43 in NFTs
In a few AD cases that did not have TDP-43 immunoreactive neuronal inclusions or neurites similar to FTLD-U, a small subset of NFTs in the subiculum and entorrhinal cortex had TDP-43 immunoreactivity. Previous studies have shown that a subpopulation of NFTs contain RNA.33 Given that TDP-43 is an RNA binding protein, one might speculate that TDP-43 binds to RNA within these lesions. Co-localization of TDP-43 immunoreactivity with acridine orange histofluorescence, which has been used to detect RNA in tissue sections,33 might shed light on this hypothesis.
The presence of TDP-43 immunoreactivity in NFTs correlated with Braak NFT stage, while TDP-43 in FTLD-U like neuronal inclusions and neurites did not. An interesting parallel with this phenomenon is the concurrence of α-synuclein and phospho-tau in the amygdala in AD. In this anatomical region, tau and α-synuclein can be found within the same neuron as separate inclusions (i.e. Lewy body and NFT) or as hybrid lesions with intermingling of α-synuclein and tau filaments.34 Interestingly, the frequency of α-synuclein in NFTs in the amygdala is also correlated with the density and severity of NFTs.13 Taken together the results suggest the possibility that tau may trigger deposition not only α-synuclein, but also TDP-43 in selectively vulnerable neuronal populations. Biochemical studies have shown synergy between tau and α-synuclein in fibril formation,35 but no such studies have been reported for TDP-43. In fact, it is unknown if TDP-43 can form fibrils.
TDP-43 in filamentous inclusions
Double labeling confocal microscopy showed that TDP-43 co-localized with phospho-tau in about 40% of neurons in AD, but in only a minority of cases was there complete overlap of phospho-tau and TDP-43 within the same inclusion. When there was co-localization, more often TDP-43 was present in a neuron with a NFT, but distinct from the NFT, either forming a separate inclusion or a separate, but intermingled, structure within the neuronal cytoplasm. Immunoelectron microscopic studies confirmed these observations in that TDP-43 immunoreactivity was most often present on 15- to 20-nm diameter filaments, dense granular material or granule-coated filaments that were distinct from tau filaments in NFTs. A few NFTs had TDP-43 immunoreactivity associated with granular material that coated the tau filaments. It was not possible to determine unequivocally if TDP-43 was associated with tau filaments or the granular material. Whether TDP-43 actually forms filaments awaits future in vitro filament assembly studies with recombinant TDP-43 or demonstration of TDP-43 immunoreactivity in filaments isolated from the brain.
The specificity of TDP-43 for FTLD-U
TDP-43 is present not only in the neuronal inclusions in FTLD-U, but also in inclusions in ALS.11, 12 TDP-43 has not been associated with neuronal or glial lesions in a host of other neurodegenerative disorders, except for a single unconfirmed report of TDP-43 immunoreactivity in Pick's disease.12 In the present study, TDP-43 immunoreactivity was not detected in progressive supranuclear palsy, corticobasal degeneration, Lewy body disease, Huntington's disease or intranuclear hyaline inclusion disease.
Concluding comments
The implications of these findings are considerable. If one accepts the specificity of TDP-43 as a marker for FTLD-U, the findings suggest that FTLD-U may be more frequent than previously suspected. The overall frequency of TDP-43 immunoreactivity in the 167 AD cases with or without HpScl was 37% (23% for AD without HpScl). If this represents concurrent FTLD-U and one extrapolates to the prevalence of AD (estimated to be ∼5 million36), then there may be more than 1 million cases of currently unrecognized mixed AD/FTLD-U.
The present study did not address the clinical features of AD with TDP-43 immunoreactivity. Almost all cases had advanced Alzheimer type pathology. If studies of Lewy body dementia are any indication, when there is mixed pathology, the greater the degree of concurrent AD pathology, the less likely the patient will present with a non-Alzheimer clinical syndrome 37 Additional studies are needed to address the clinical significance of TDP-43 immunoreactive pathology in AD.
Acknowledgments
Supported by NIH grants P50-AG25711, P50-AG16574, P50-NS40256, P01-AG17216 and P01-AG03949. The histological support of Virginia Phillips, Linda Rousseau and Monica Casey-Castanedes is greatly appreciated. The assistance of John Gonzalez with biochemical studies is also acknowledged. Most of the cases used in this study were derived from the State of Florida Alzheimer Disease Initiative brain bank funded by the State of Florida Department of Elder Affairs.
REFERENCES
- 1.McKhann GM, Albert MS, Grossman M, et al. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Arch Neurol. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- 2.Bergmann M, Kuchelmeister K, Schmid KW, et al. Different variants of frontotemporal dementia: a neuropathological and immunohistochemical study. Acta Neuropathol (Berl) 1996;92:170–179. doi: 10.1007/s004010050505. [DOI] [PubMed] [Google Scholar]
- 3.Lipton AM, White CL, 3rd, Bigio EH. Frontotemporal lobar degeneration with motor neuron disease-type inclusions predominates in 76 cases of frontotemporal degeneration. Acta Neuropathol (Berl) 2004;108:379–385. doi: 10.1007/s00401-004-0900-9. [DOI] [PubMed] [Google Scholar]
- 4.Mann DM, South PW, Snowden JS, Neary D. Dementia of frontal lobe type: neuropathology and immunohistochemistry. J Neurol Neurosurg Psychiatry. 1993;56:605–614. doi: 10.1136/jnnp.56.6.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snowden JS, Neary D, Mann DM. Frontotemporal dementia. Br J Psychiatry. 2002;180:140–143. doi: 10.1192/bjp.180.2.140. [DOI] [PubMed] [Google Scholar]
- 6.Tolnay M, Probst A. Frontal lobe degeneration: novel ubiquitin-immunoreactive neurites within frontotemporal cortex. Neuropathol Appl Neurobiol. 1995;21:492–497. doi: 10.1111/j.1365-2990.1995.tb01095.x. [DOI] [PubMed] [Google Scholar]
- 7.Okamoto K, Murakami N, Kusaka H, et al. Ubiquitin-positive intraneuronal inclusions in the extramotor cortices of presenile dementia patients with motor neuron disease. J Neurol. 1992;239:426–430. doi: 10.1007/BF00856806. [DOI] [PubMed] [Google Scholar]
- 8.Wightman G, Anderson VE, Martin J, et al. Hippocampal and neocortical ubiquitin-immunoreactive inclusions in amyotrophic lateral sclerosis with dementia. Neurosci Lett. 1992;139:269–274. doi: 10.1016/0304-3940(92)90569-s. [DOI] [PubMed] [Google Scholar]
- 9.Cairns NJ, Tu P-H, Bigio E, et al. Neuropathologic heterogeneity in FTLD with ubiquitin inclusions: report of the Midwest Consortium for FTLD. Brain Pathol. 2006;16(Suppl 1):S5. [Google Scholar]
- 10.Hamilton RL, Bowser R. Alzheimer disease pathology in amyotrophic lateral sclerosis. Acta Neuropathol (Berl) 2004;107:515–522. doi: 10.1007/s00401-004-0843-1. [DOI] [PubMed] [Google Scholar]
- 11.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 12.Arai T, Hasegawa M, Akiyama H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 13.Uchikado H, Lin WL, DeLucia MW, Dickson DW. Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy. J Neuropathol Exp Neurol. 2006;65:685–697. doi: 10.1097/01.jnen.0000225908.90052.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lippa CF, Dickson DW. Hippocampal sclerosis dementia: expanding the phenotypes of frontotemporal dementias? Neurology. 2004;63:414–415. doi: 10.1212/01.wnl.0000136241.71716.72. [DOI] [PubMed] [Google Scholar]
- 15.Josephs KA, Dickson DW. Hippocampal sclerosis in tau-negative frontotemporal lobar degeneration. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Knopman DS, Mastri AR, Frey WH, 2nd, et al. Dementia lacking distinctive histologic features: a common non-Alzheimer degenerative dementia. Neurology. 1990;40:251–256. doi: 10.1212/wnl.40.2.251. [DOI] [PubMed] [Google Scholar]
- 17.Barker WW, Luis CA, Kashuba A, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. 2002;16:203–212. doi: 10.1097/00002093-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Togo T, Cookson N, Dickson DW. Argyrophilic grain disease: neuropathology, frequency in a dementia brain bank and lack of relationship with apolipoprotein E. Brain Pathol. 2002;12:45–52. doi: 10.1111/j.1750-3639.2002.tb00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jicha GA, Weaver C, Lane E, et al. cAMP-dependent protein kinase phosphorylations on tau in Alzheimer's disease. J Neurosci. 1999;19:7486–7494. doi: 10.1523/JNEUROSCI.19-17-07486.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenberg SG, Davies P. A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1990;87:5827–5831. doi: 10.1073/pnas.87.15.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santacruz K, Lewis J, Spires T, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin WL, Lewis J, Yen SH, et al. Filamentous tau in oligodendrocytes and astrocytes of transgenic mice expressing the human tau isoform with the P301L mutation. Am J Pathol. 2003;162:213–218. doi: 10.1016/S0002-9440(10)63812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beach TG, Sue L, Scott S, et al. Hippocampal sclerosis dementia with tauopathy. Brain Pathol. 2003;13:263–278. doi: 10.1111/j.1750-3639.2003.tb00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackenzie IR, Baborie A, Pickering-Brown S, et al. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol (Berl) 2006 doi: 10.1007/s00401-006-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sampathu DM, Neumann M, Kwong LK, et al. Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol. 2006;169:1343–1352. doi: 10.2353/ajpath.2006.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katsuse O, Dickson DW. Ubiquitin immunohistochemistry of frontotemporal lobar degeneration differentiates cases with and without motor neuron disease. Alzheimer Dis Assoc Disord. 2005;19(Suppl 1):S37–43. doi: 10.1097/01.wad.0000183889.61421.a8. [DOI] [PubMed] [Google Scholar]
- 27.Kosaka K, Tsuchiya K, Yoshimura M. Lewy body disease with and without dementia: a clinicopathological study of 35 cases. Clin Neuropathol. 1988;7:299–305. [PubMed] [Google Scholar]
- 28.Kinoshita A, Tomimoto H, Suenaga T, et al. Ubiquitin-related cytoskeletal abnormality in frontotemporal dementia: immunohistochemical and immunoelectron microscope studies. Acta Neuropathol (Berl) 1997;94:67–72. doi: 10.1007/s004010050673. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto K, Hirai S, Yamazaki T, et al. New ubiquitin-positive intraneuronal inclusions in the extra-motor cortices in patients with amyotrophic lateral sclerosis. Neurosci Lett. 1991;129:233–236. doi: 10.1016/0304-3940(91)90469-a. [DOI] [PubMed] [Google Scholar]
- 30.Hatanpaa KJ, Blass DM, Pletnikova O, et al. Most cases of dementia with hippocampal sclerosis may represent frontotemporal dementia. Neurology. 2004;63:538–542. doi: 10.1212/01.wnl.0000129543.46734.c0. [DOI] [PubMed] [Google Scholar]
- 31.Ishizawa T, Mattila P, Davies P, et al. Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol. 2003;62:389–397. doi: 10.1093/jnen/62.4.389. [DOI] [PubMed] [Google Scholar]
- 32.Duda JE, Giasson BI, Mabon ME, et al. Concurrence of alpha-synuclein and tau brain pathology in the Contursi kindred. Acta Neuropathol (Berl) 2002;104:7–11. doi: 10.1007/s00401-002-0563-3. [DOI] [PubMed] [Google Scholar]
- 33.Ginsberg SD, Crino PB, Lee VM, et al. Sequestration of RNA in Alzheimer's disease neurofibrillary tangles and senile plaques. Ann Neurol. 1997;41:200–209. doi: 10.1002/ana.410410211. [DOI] [PubMed] [Google Scholar]
- 34.Iseki E, Marui W, Kosaka K, Ueda K. Frequent coexistence of Lewy bodies and neurofibrillary tangles in the same neurons of patients with diffuse Lewy body disease. Neurosci Lett. 1999;265:9–12. doi: 10.1016/s0304-3940(99)00178-0. [DOI] [PubMed] [Google Scholar]
- 35.Giasson BI, Forman MS, Higuchi M, et al. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science. 2003;300:636–640. doi: 10.1126/science.1082324. [DOI] [PubMed] [Google Scholar]
- 36.Hebert LE, Scherr PA, Bienias JL, et al. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 37.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]