Abstract
Orexin-A (ORXA) is an orexigenic neuropeptide produced by the lateral hypothalamus that increases food intake when injected into the brain ventricles or forebrain nuclei. We used a licking microstructure analysis to evaluate hindbrain and forebrain ORXA effects in intact and hindbrain-lesioned rats, to identify the motivational and anatomical bases of ORXA hyperphagia. Intact rats with cannulas in the fourth brain ventricle (4V) received vehicle (artificial cerebrospinal fluid) or ORXA (0.1, 0.4, 1, or 10 nm) injections before 90 min access to 0.1 m sucrose. Meal size and frequency were increased in a double-dissociated manner by the 1 and 10 nm doses, respectively. In experiment 2, 4V 1 nm ORXA was applied to rats offered solutions varied in caloric and gustatory intensity (water and 0.1 and 1 m sucrose). ORXA increased meal frequency for all tastants. ORXA increased meal size only for 0.1 m sucrose, by prolonging the meal without affecting early ingestion rate or lick burst size, suggesting that 4V ORXA influenced inhibitory postingestive feedback rather than taste evaluation. In experiment 3, rats with cannulas in the third ventricle (3V) received dorsal medullary lesions centered on the area postrema (APX group) or sham procedures, and licking for water and 0.1 and 1 m sucrose was evaluated after 1 nm 3V ORXA/artificial cerebrospinal fluid injections. The 3V ORXA increased 0.1 m sucrose meal size and meal frequency for all tastants in the sham group, as observed after 4V ORXA in experiment 2. In the APX group, 3V ORXA injections influenced meal frequency, but they no longer increased meal size. However, the APX rats increased meal size for 0.1 m sucrose after food and water deprivation and after 3V angiotensin II injection. They also showed meal size suppression after 3V injection of the melanocortin-3/4 receptor agonist melanotan II (1 nm). These findings suggest that the area postrema and subjacent nucleus of the solitary tract are necessary for increases in consummatory (meal size) but not appetitive (meal frequency) responses to 3V ORXA. The meal size increases may be due to reduced postingestive feedback inhibition induced by ORXA delivered to either the hindbrain or forebrain ventricles.
Orexin-A increases meal size via gut feedback disinhibition, not enhanced gustatory evaluation. Medullary lesions abolish meal size but not frequency increases, indicating dissociable sites for orexin-A feeding actions.
Orexin-A (ORXA) is an orexigenic hypothalamic neuropeptide implicated in feeding, drinking, waking, activity, reward, and gastrointestinal processing (1,2,3,4,5,6,7). Studies have shown that forebrain intracerebroventricular (icv) and hypothalamic injections of ORXA reliably increase intake of food, water, saccharin, ethanol, and sucrose solutions (1,5,8,9,10,11,12,13,14,15). Neurons that synthesize ORXA, along with those that synthesize another orexigenic peptide, melanin-concentrating hormone (MCH), are limited to lateral hypothalamic (LH), dorsomedial hypothalamic, and zona incerta regions (perifornical and juxtacapsular zones), although they project extensively throughout the brain (11,16,17,18,19,20). Both peptides are considered potentially important in the mediation of hypothalamic influences on feeding and metabolism (11,21,22,23,24,25), because both are sensitive to and reciprocally connected with the neuropeptide Y (NPY)- and proopiomelanocortin (POMC)-synthesizing neurons of the arcuate nucleus, which in turn are responsive to hormones that influence metabolism, food intake, and body weight such as ghrelin and leptin (16,24,26,27,28,29,30). Several forebrain sites have been identified as loci of action for these orexigenic peptides. For example, ORXA immunoreactive fibers or orexin-1 and/or orexin-2 receptor mRNA have been identified in the anterior, arcuate, paraventricular, dorsomedial, ventromedial, lateral and perifornical hypothalamic nuclei (16,31,33,35), the ventral tegmental area (33,35), nucleus accumbens (33), and bed nucleus of the stria terminalis.
A growing body of evidence suggests that the actions of hypothalamic neuropeptides are distributed throughout the brain such that various aspects of ingestive behavior may be differentially affected by different sites of neuropeptide action. We recently characterized differences in feeding after hindbrain [fourth ventricle (4V)] and forebrain [third ventricle (3V)] infusions of NPY and MCH using a detailed microstructure analysis of feeding behaviors (32). NPY infusions increased consummatory feeding (meal size) for sucrose solutions, whether it was applied to the 3V or the 4V. The meal size increase was mediated by a pattern of ingestive behaviors consistent with a reduction of inhibitory gastrointestinal feedback (satiation). NPY also increased appetitive feeding (meal frequency), but this was observed only after the 3V infusion. In contrast with NPY, MCH had no effect in the hindbrain, because sucrose meal size (consummatory feeding) was increased only when applied to the 3V. Furthermore, the 3V MCH infusions increased meal size through influences on taste evaluation rather than satiation, and there was no effect on meal frequency. The failure of 4V MCH to influence any measures of feeding microstructure led us to explore the role of the LH colocalized orexigen ORXA on the behavioral processes underlying consumption after 3V and 4V ORXA infusions.
Although MCH and ORXA are colocalized to the LH (17,18), and both produce hyperphagia, several findings suggest that ORXA increases food intake through different sites of action and feeding processes relative to those affected by MCH. First, contrary to MCH, ORXA infusions to the 4V were reported to increase chow consumption, although direct ORXA infusions to the nucleus of the solitary tract (NST) showed no effect on chow intake, little effect on sucrose intake, and only moderate effects on high-fat diet intake (11,25,31). Second, previous studies suggest that ORXA infusions affect gastrointestinal motility through direct actions in the hindbrain (2) and therefore may increase food intake by suppressing gastrointestinal satiation signals, distinct from MCH, which influences taste evaluation rather than satiation (32).
The goals of this study were 3-fold. First, we evaluated whether the behavioral processes underlying the previously documented orexigenic effects of forebrain ORXA infusions were qualitatively and/or quantitatively different when the infusions were restricted to the hindbrain. To that end, we evaluated the effects of 4V ORXA infusions on licking for water and sucrose (0.1 and 1 m) solutions, which provided a range of taste and calorie intensities against which ORXA effects on taste evaluation and postingestive sensitivity (satiation) could be assayed. We also determined ORXA effects on appetitive feeding (meal frequency) vs. consummatory feeding (measured by meal size and licking microstructure), based on evidence that ORXA increases appetitive (food-seeking) behavior. Second, we examined the differences between responses to forebrain (3V) vs. hindbrain (4V) ORXA through a comparison with responses to the same taste solutions after a comparable ORXA dose delivered to the 3V. Third, to further pinpoint the anatomical sites of ORXA feeding action, we evaluated 3V ORXA responses for the same taste solutions after the area postrema (AP), and portions of the underlying NST were lesioned. These sites have been shown to receive direct input from approximately 20% of the ORXA neurons in the LH (11), with dense immunoreactive fiber labeling in the AP (68) and strong levels of fos protein after ORXA infusion (11,68). Orexin-1 receptor mRNA has been identified in NST and dorsal motor nucleus of the vagus (DMX) neurons (33,35,36). Although data for the AP were not reported in these studies, it is worth noting that the dendrites of some NST neurons pervade the AP (37), and electron microscopic analysis revealed ORXA immunoreactive product in presynaptic axon terminals of axosomatic and axodendritic synapses within the AP (68). Furthermore, patch clamp results showed that dissociated AP neurons were directly depolarized by ORXA (38). These findings suggest that ORXA is released and binds to receptors within the AP.
Materials and Methods
Animals
Adult albino male Sprague Dawley rats (Charles River, Wilmington, MA) weighing 184–368 g on the first day of the main experiments were used. Test groups were more closely age and weight matched within each experiment. The lesion groups required more preparation before the experiment, resulting in greater body weight at testing. Rats were maintained individually in plastic tubs (48 × 25 × 15 cm) with wire lids on a 12-h light, 12-h dark schedule in a temperature-controlled room. Food (Purina rat chow 5001; Lab Diets, St. Louis, MO) and tap water were available ad libitum in the home cage, except where noted below. Rats were tested at the same time each day, between 4 and 8 h after lights on (0700 h), in a separate test cage.
Surgery
All procedures were approved by the Amherst College Institutional Animal Care and Use Committee. Rats were anesthetized (ip) with a mixture of ketamine HCl (66 mg/kg) and xylazine HCl (6 mg/kg). A 22-gauge guide cannula (Plastics One, Roanoke, VA) was stereotaxically implanted into either the 3V (from bregma: anteroposterior −2.3 mm, mediolateral 0 mm, and dorsoventral −8.5 mm from skull surface) or 4V (from lambda: anteroposterior −3.2 mm, mediolateral 0 mm, and dorsoventral −7.2 mm from skull surface) and fastened with dental acrylic and skull screws. The 28-gauge injection cannula extended 1 mm below the tip of the guide cannula, and a dummy cannula cut flush to the guide tip was maintained in the guide cannula at all other times. After surgical recovery, correct placement of the 4V cannula was confirmed by an increase in blood glucose after a cannula infusion of the antimetabolite, 5-thio-d-glucose (120 μg/2 μl; 2 μl/min) (39). Tail blood was collected every 10 min starting 20 min before the 5-thio-d-glucose injection, using a commercially available glucometer and test strips. Rats that did not exhibit at least a 50% increase in blood glucose within 30 min of injection were removed from the study. Correct placement for the 3V cannula was confirmed by a minimum of 5 ml water consumption within 30 min of a 50 ng/5 μl cannula injection of angiotensin II (32,40,41).
At least 2 d after 3V cannula placement confirmation, rats were anesthetized a second time and received either an AP/NST lesion (APX group) or sham procedures. The surgery was done after cannula patency confirmation to minimize unnecessary surgery in rats that could not be tested and to maximize their viability to provide pilot data for unrelated experiments. The anesthetized rat was placed in the stereotaxic apparatus with the head flexed ventral to maximize exposure of the brainstem. The dorsal medullary surface was exposed by retracting the overlying neck muscles and removing the posterior atlanto-occipital membrane. Using a surgical microscope (Seiler M900), the floor of the 4V and the region containing the AP was identified. The AP was carefully aspirated using a PE-10 tubing nozzle attached via larger PE and Tygon tubing segments connected to a vacuum pump. The access hole through the meninges and membrane was then carefully filled with gel foam, the neck muscles and scalp were sutured, and the rat was allowed to recover from the operation. Sham-lesioned rats were treated identically with the exception that the aspirator was not introduced near the AP.
Concluding behavioral experiments, cannula placements were again confirmed; India ink (2 μl) was injected immediately after a lethal overdose of sodium pentobarbital (100 mg/kg). Rats were then transcardially perfused with isotonic saline followed by 10% formalin. The brain was removed, and the whole brain was bisected midsagittally in 4V cannula fitted rats, whereas the forebrain was blocked and bisected in 3V cannula fitted rats, and both sets of brains were inspected for ventricular ink perfusion. Data for rats with no ink perfusion of the 3V or 4V were discarded. Ink was never observed to have reached the third or lateral ventricles after a 4V infusion. The hindbrain sections of the 3V fitted rats (which had also received APX/SHAM surgery) were then preserved 1–3 d in a 20% sucrose-formalin solution and cut in to 50-μm serial sections, mounted on microscope slides, and then stained using neutral red for analysis of the extent of lesion using bright-field microscopy at ×40 and ×100 magnification.
Apparatus
Rats were taken from their home cages and tested in individual plastic tubs (48 × 25 × 15 cm). A drinking spout (3-mm orifice; Girton Inc., Millville, PA) was introduced to the test chamber with the spout orifice positioned 4 cm from the floor and 0–1 mm behind a slit (8 × 28 mm) in a metal plate attached to the front of the cage. A lickometer (MS-108; DiLog Instruments, Tallahassee, FL) and PC were used to record licking; tongue contacts with the spout completed a circuit, which allowed the computer to record the time of each lick with 1-msec resolution. Files for each test session for each rat were saved for offline analysis.
Procedures
Before testing, rats in all experiments were habituated in the test cage daily where they were free to ingest 0.5 m sucrose solution for 90 min. Habituation training continued until session intakes stabilized and exceeded 5 ml per session (two to five sessions).
Experiment 1: 4V ORXA dose-response analysis
Rats were offered 0.1 m sucrose once daily for 90 min. On d 3, 6, 9, 12, and 15, rats received a 2-μl 4V injection (1 μl/min) of either vehicle [artificial cerebrospinal fluid (aCSF); Harvard Apparatus, Holliston, MA], or 0.1, 0.4, 1.0, or 10 nm human ORXA (American Peptide, Sunnyvale, CA) in counterbalanced order 15 min before behavioral testing. The 10 nm dose corresponds to a moderate icv ORXA dose shown to increase food intake (most studies reporting ranges from 1–30 nm ORXA) (4). The 2-min infusions were performed using a 10-μl Hamilton syringe in a programmable syringe pump (KD Scientific, Holliston, MA model 100).
Experiment 2: 4V ORXA concentration-response analysis
To assess the influence of 4V ORXA on gustatory and postingestive sensitivity, rats were offered each of three taste solutions: water, 0.1 m sucrose, and 1 m sucrose. These solutions produce systematic differences in licking and consumption responses. Water is noncaloric, less palatable, and usually less consumed than either sucrose solution (32,41). The 0.1 and 1 m sucrose solutions often produce distinct licking microstructure profiles even though these differences can result in the same volume consumed (32,41,42). Because there is little postingestive accumulation of food at the beginning of a meal, the initial rate of ingestion is influenced primarily by orosensory stimulus properties (taste). We have shown that the 1 m sucrose solution generates robust gustatory responses and is avidly consumed, resulting in a high initial rate of ingestion and larger mean lick burst sizes (reflecting taste evaluation), and a steeper slope of decline in ingestion rate due to the caloric impact of the solution. The less caloric and less preferred 0.1 m sucrose solution yields slower initial ingestion rates and smaller licking bursts (marking weaker hedonic taste evaluation) and a flatter slope of decline in ingestion rate, with a longer meal duration and more bursts of licking, reflecting the reduced inhibitory impact of the postingestive load due to fewer calories in the solution (see also Refs. 42 and 43 for further discussion).
Rats were exposed to the same taste solution for 90 min daily over 5 consecutive test days, with 4V injections on d 3 and 5 of each concentration block. Two nontest days intervened each of the three 5-d concentration test blocks. The three concentration blocks and drug order with those blocks were counterbalanced with a Latin square. On drug test days, rats received a 1 nm/2 μl cannula injection (1 μl/min) of either ORXA or aCSF as performed in experiment 1. This dose corresponded to the most hyperphagic dose for meal size identified in experiment 1.
Experiment 3: role of the AP/NST in ORXA hyperphagia
To evaluate the contributions of the AP/NST to ORXA hyperphagia, rats in this study were fitted with 3V cannulas and received either APX or sham surgery before testing. The study design was otherwise identical to experiment 2.
Experiment 4: role of the AP/NST in hyper- and hypophagic responses to other stimuli
After testing the first cohort of APX rats in experiment 3, we appended a control condition to the study for the remaining rats tested. Thus, immediately after completion of the main experiment, APX rats received 0.1 m sucrose for 3 d. Fifteen minutes before testing on the third test day, rats were injected with 1 nm/2 μl of the melanocortin-3/4 receptor agonist melanotan II (MTII; American Peptide, Sunnyvale, CA).
A second group of rats with APX lesions and 3V cannulas was also tested for their responses to 22.5 h food and water deprivation and to angiotensin II. These rats had previously received 3V NPY injections (1 nm) in an unrelated experiment and weighed 284–425 g at testing. The rats had previous training with sucrose and were thus offered 0.1 m sucrose for 90 min daily in the lickometer for 2–3 consecutive days. For half of the rats, food and water were then removed from the home cage 22.5 h before offering them 0.1 m sucrose for 90 min on the next day. Food and water was returned thereafter, and the rats were given 2–3 more days to consume 0.1 m sucrose. On the next day, these rats received a 3V injection of angiotensin II (50 ng/5 μl) 15 min before 90 min access to hypotonic 0.1 m sucrose. The other half of the rat group was tested in reverse order.
Data analysis
Data were analyzed according to previously established analysis parameters, as follows (see Refs. 40,41, and 44 for details). Microstructure analyses were limited to the first meal in the test session.
Meal size (lick count) was calculated as the number of licks in the meal (first lick of the first burst to last lick of the last burst) (40,45). The end of the meal was defined by a pause in licking greater than or equal to 10 min (44). Meal duration (minutes) was defined as the session time of the last lick in the meal minus the session time of the first lick in the meal. Average ingestion rate (licks per minute) was calculated as the number of licks in the meal divided by meal duration. Lick volume (microliters) was calculated by dividing the difference between pre- and posttest weights of the spout bottle by the total number of licks in the session. Meal size (milliliters) was then calculated by multiplying the meal lick count by the lick volume.
The temporal distribution of licking was analyzed using a variety of custom-made programs (40,46,47). A licking burst was defined as two or more consecutive licks with no inter-lick interval (ILI) exceeding 1 sec. Thus, pauses greater than 1 sec determined burst termination (45). Burst duration (seconds) was calculated by subtracting the session time of the first lick in the burst from the time of the last lick in that burst. Mean burst size (lick count) was calculated as the cumulative number of licks in all bursts in the meal divided by the number of bursts in the meal. To minimize artifacts due to nonlingual spout contacts, meal onset was defined as the first lick of the first burst containing at least three licks. Latency (seconds) was defined as the time between placement of the rat into the test cage and meal onset. Initial lick rate was the number of licks in the first minute of the meal.
ILIs were analyzed in several ways. The average within-burst ILI (milliseconds) was determined by averaging all ILIs less than 1 sec. Because more than 95% of all ILIs in a meal are less than 250 msec (in rats) and are normally distributed below this cutoff (44,48), the average duration of ILIs less than 250 msec was also determined. We also evaluated the relative proportion of ILIs less than 250 msec as a percentage of all ILIs within bursts (ILIs less than 1 sec). Any significant variation in this proportion indicates a reciprocal variation in the proportion of ILIs ranging from 250–999 msec.
The mean pause duration (seconds) was defined as the meal duration minus the cumulative duration of bursts in the meal, divided by the number of meal pauses (number of bursts minus one). Percent pause duration for the meal was the cumulative time of all pauses divided by the meal duration multiplied by 100.
For experiment 1, results for the five drug levels (aCSF, 0.1, 0.4, 1, and 10 nm) were analyzed with a one-way repeated-measures ANOVA using SPSS 16 software. Responses for each measure in experiments 2 and 3 across the two drug days (ORXA/aCSF) for each of the three taste solutions (water and 0.1 and 1 m sucrose) were compared with two-way (drug × tastant) repeated-measures ANOVA. Planned t tests were used to compare drug responses for each solution, to inform any significant main effects or interactions. A mixed-factors two-way ANOVA was used to assure comparable baseline conditions between lesion groups (group aCSF condition × tastant) in experiment 3 and to compare the sham group results with those obtained in experiment 2, to allow comparison of forebrain (3V) vs. hindbrain (4V) icv infusions of ORXA in intact rats. Difference scores (ORXA minus aCSF) for each rat in the 3V and 4V groups were also compared to evaluate the effect magnitudes of ORXA-induced responses. In experiment 4, intake for both treatments was compared with that on preceding baseline days or aCSF conditions using paired t tests. The criterion for a statistically significant difference was P < 0.05.
Results
Experiment 1: 4V ORXA dose-response effects
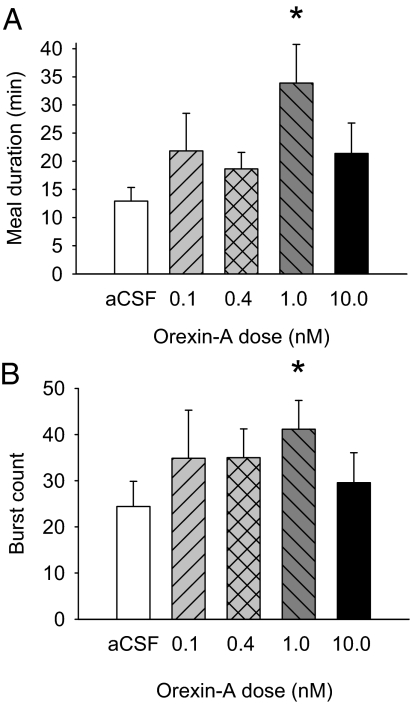
In the seven rats in this experiment, ORXA doubled intake at the two strongest doses tested, 1 and 10 nm [F(4,24) = 23.29; P = 0.003; Fig. 1A]. Interestingly, intake was increased by different underlying processes at each dose. The increase at the 1 nm dose was due primarily to an increase in first meal size, as indicated by significant ANOVA [F(4,24) = 2.83; P < 0.05] and paired t tests (Fig. 1B); however, after the 10 nm infusion, there was a stronger effect on meal frequency rather than meal size [F(4,24) = 4.08; P < 0.01; Fig. 1C].
Figure 1.
ORXA infused to the 4V increased the consumption of 0.1 m sucrose during the test session to a similar extent at the 1 and 10 nm doses, but this result was achieved through separate underlying dose-dependent processes. A, Mean (+ se) 90-min intake (milliliters) values for aCSF (white bar) and four doses of ORXA (hatched and black bars) in 4V cannula-fitted rats (n = 7) ingesting 0.1 m sucrose solution. *, P < 0.05. B, Mean (+se) meal size (milliliters) for the same conditions as in A. *, P < 0.05. C, Mean (+se) number of meals initiated for the test solution for the same conditions as in A. *, P < 0.05.
The microstructure changes after ORXA suggested that 1 nm ORXA increased meal size by suppressing inhibitory postingestive feedback. First, ORXA did not significantly influence either the initial lick rate or the mean burst size, the two measures commonly associated with gustatory evaluation (Table 1). Second, neither lick volume nor any of the ILI measures were affected (Table 1), indicating that ORXA did not influence oromotor coordination or the intrinsic rate of licking within bursts. ORXA also had no influence on latency to begin feeding (Table 1). Rather, ORXA significantly prolonged the meal as indicated by a statistically significant increase in meal duration (Fig. 2A). The mean burst count at 1 nm was also significantly increased relative to aCSF (Fig. 2B).
Table 1.
Licking measures across drug dose conditions
| Measure | 4V ORXA dose
|
ANOVA, dose F(4,24) (P) | ||||
|---|---|---|---|---|---|---|
| aCSF | 0.1 nm | 0.4 nm | 1.0 nm | 10 nm | ||
| Meal lick count | 1400 ± 206 | 2023 ± 377 | 1905 ± 297 | 2786 ± 463 | 1876 ± 269 | 3.96 (0.01) |
| Latency (sec) | 30.19 ± 12.01 | 208.40 ± 192.66 | 24.09 ± 8.09 | 24.83 ± 6.44 | 29.07 ± 7.84 | 0.87 (NS) |
| First minute lick rate | 251 ± 46 | 263 ± 52 | 244 ± 29 | 226 ± 34 | 161 ± 44 | 1.43 (NS) |
| Mean burst size (licks) | 68.32 ± 9.99 | 116.86 ± 50.45 | 57.30 ± 4.78 | 72.91 ± 10.66 | 78.64 ± 18.09 | 0.85 (NS) |
| Mean lick rate (licks/min) | 120.70 ± 19.03 | 155.98 ± 43.05 | 113.44 ± 17.43 | 89.82 ± 9.84 | 120.35 ± 23.60 | 0.94 (NS) |
| Pause time (% meal duration) | 67.19 ± 4.57 | 70.60 ± 5.52 | 74.61 ± 3.39 | 76.84 ± 2.71 | 69.03 ± 6.42 | 0.91 (NS) |
| Lick volume (μl) | 5.80 ± 0.33 | 5.74 ± 0.39 | 5.58 ± 0.42 | 5.72 ± 0.25 | 6.23 ± 0.43 | 1.43 (NS) |
| Within-burst lick rate (licks/sec) | 6.5 ± 0.2 | 6.5 ± 0.1 | 6.4 ± 0.1 | 6.7 ± 0.1 | 6.7 ± 0.2 | 1.55 (NS) |
| Mean ILI (0–249 msec) | 145.75 ± 4.35 | 146.51 ± 3.76 | 147.91 ± 2.67 | 143.27 ± 3.67 | 145.09 ± 4.19 | 0.93 (NS) |
| ILI% (0–249 msec) | 97.13 ± 0.51 | 96.56 ± 0.89 | 97.78 ± 0.58 | 98.28 ± 0.39 | 98.63 ± 0.26 | 1.53 (NS) |
Results are shown as mean ± se. Statistical significance was set at P≤ 0.05. Significant F/P ANOVA values appear in boldface. ILI%, Proportion of ILIs in the range indicated relative to the total of all ILIs less than 1 sec.
Figure 2.
The increase in meal size induced by 4V 1 nm ORXA was achieved through increases in the meal duration and the number of licking bursts in the meal. A, Mean (+se) meal duration (minutes) values for aCSF (white bar) and four doses of ORXA (hatched and black bars) in 4V cannula-fitted rats (n = 7) ingesting 0.1 m sucrose solution [ANOVA: F(4,24) = 2.99, P < 0.04; aCSF-1 nm comparison: *, P < 0.05]. B, Mean (+se) number of bursts in the meal for the same conditions as in A [ANOVA: F(4,24) = 1.09, NS; aCSF-1 nm comparison: *, P < 0.04].
Experiment 2: 4V ORXA effects on licking microstructure for a range of taste solutions
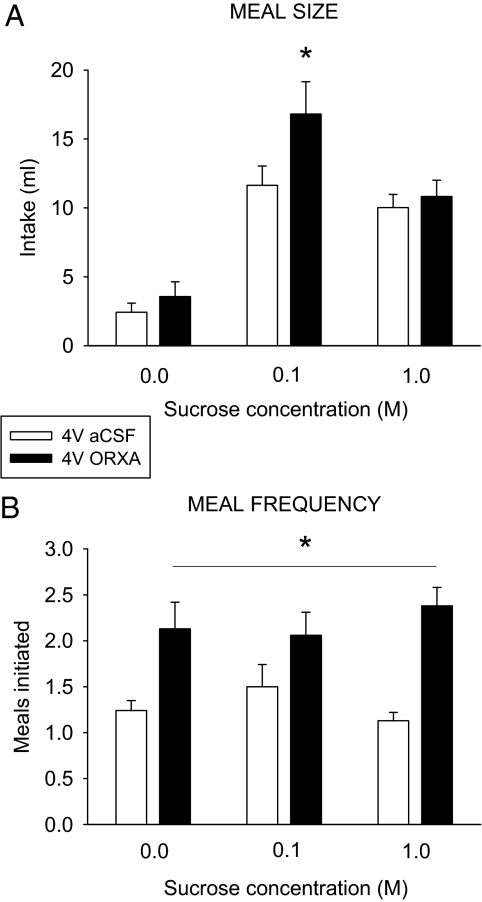
For the 16 rats in this experiment, meal size under baseline conditions varied across tastants, as anticipated (32,41,42,44) (Fig. 3A). As observed in experiment 1, ORXA significantly increased meal size; however, this result was observed only for the 0.1 m sucrose solution (Fig. 3A). Interestingly, ORXA influenced appetitive feeding (meal frequency) in a manner dissociated from its influence on consummatory feeding (meal size), because the meal frequency was increased for all three taste solutions (Fig. 3B). This appetitive effect resulted in significant increases for overall intake for the total test session for both sucrose solutions, although the effect was most pronounced for 0.1 m sucrose (Table 2), where the increases in consummatory and appetitive feeding synergized.
Figure 3.
ORXA (1 nm) infused to the 4V differentially increased the meal size or meal frequency in a concentration-dependent manner. A, Mean (+ se) meal size (milliliters) values after aCSF (white bar) and ORXA (black bars) injections in 4V cannula-fitted rats (n = 16) ingesting water and 0.1 and 1 m sucrose solutions [ANOVA: concentration F(2,30) = 26.49, P < 0.001; drug F(1,15) = 8.74, P < 0.01; interaction F(2,30) = 5.26, P < 0.01; 0.1 m pairwise comparison: t(15) = −3.22; *, P = 0.006]. B, The mean (+se) number of meals initiated in the test session for the same conditions as in A did not vary by tastant concentration [F(2,30) = 0.12, NS], but it was increased for all tastants after ORXA [drug F(1,15) = 43.95; *, P < 0.001; interaction F(2,30) = 1.25, NS].
Table 2.
Licking measures across tastant and drug conditions
| Measure | Water | 0.1 m sucrose | 1 m sucrose | ANOVA
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| aCSF | 3V ORXA | aCSF | 3V ORXA | aCSF | 3V ORXA | Drug F(1,15) (P) | Concentration F(2,30) (P) | Interaction F(2,30) (P) | |
| 90-min intake (ml) | 2.78 ± 0.62 | 4.92 ± 1.16 | 13.19 ± 2.03 | 21.21 ± 3.30 | 10.39 ± 0.96 | 15.13 ± 1.46 | 33.75 (0.001) | 21.13 (0.001) | 5.65 (0.008) |
| Meals initiated | 1.25 ± 0.11 | 2.13 ± 0.29 | 1.50 ± 0.24 | 2.06 ± 0.25 | 1.13 ± 0.09 | 2.38 ± 0.20 | 43.95 (0.001) | 0.12 (NS) | 1.25 (NS) |
| Meal lick count | 426 ± 121 | 630 ± 202 | 2122 ± 286 | 2957 ± 412 | 1635 ± 144 | 1802 ± 170 | 8.62 (0.01) | 22.66 (0.001) | 5.38 (0.01) |
| Latency (sec) | 98.4 ± 35.5 | 464.8 ± 232.8 | 18.5 ± 4.5 | 296.8 ± 268.9 | 9.40 ± 1.7 | 9.3 ± 1.1 | 1.89 (NS) | 2.65 (NS) | 1.48 (NS) |
| First minute lick rate | 96 ± 35 | 187 ± 42 | 272 ± 28 | 272 ± 24 | 286 ± 19 | 287 ± 17 | 2.10 (NS) | 12.70 (0.001) | 2.43 (NS) |
| Mean burst size (licks) | 44.4 ± 9.9 | 52.8 ± 9.8 | 67.9 ± 10.8 | 71.6 ± 7.5 | 98.1 ± 12.7 | 91.5 ± 11.3 | 0.17 (NS) | 8.80 (0.001) | 0.49 (NS) |
| Mean lick rate (licks/min) | 150.0 ± 32.6 | 157.0 ± 28.4 | 115.5 ± 15.3 | 118.3 ± 19.0 | 204.9 ± 17.9 | 184.8 ± 16.8 | 0.07 (NS) | 5.02 (0.006) | 0.40 (NS) |
| Mean pause duration (sec) | 49.1 ± 16.1 | 34.9 ± 8.1 | 24.0 ± 3.0 | 28.7 ± 5.2 | 16.2 ± 1.6 | 20.0 ± 5.0 | 0.18 (NS) | 6.40 (0.01) | 0.66 (NS) |
| Pause time (%) | 63.8 ± 8.6 | 56.8 ± 9.5 | 70.4 ± 3.7 | 68.6 ± 5.4 | 51.2 ± 4.2 | 54.0 ± 4.5 | 1.03 (NS) | 2.57 (NS) | 0.51 (NS) |
| Lick volume (μl) | 7.56 ± 1.13 | 7.97 ± 1.87 | 5.70 ± 0.18 | 5.97 ± 0.52 | 6.16 ± 0.24 | 5.93 ± 0.23 | 0.008 (NS) | 4.04 (0.03) | 0.01 (NS) |
| Within-burst lick rate (licks/sec) | 5.2 ± 0.4 | 5.5 ± 0.3 | 6.3 ± 0.1 | 6.4 ± 0.1 | 6.4 ± 0.1 | 6.5 ± 0.1 | 1.21 (NS) | 12.19 (0.001) | 0.29 (NS) |
| Mean ILI (0–249 msec) | 150.5 ± 6.77 | 145.4 ± 2.5 | 144.8 ± 2.1 | 146.0 ± 3.1 | 146.3 ± 2.4 | 145.8 ± 2.3 | 0.35 (NS) | 0.34 (NS) | 0.63 (NS) |
| ILI% (0–249 msec) | 81.2 ± 6.5 | 88.2 ± 4.4 | 96.3 ± 0.7 | 97.4 ± 0.5 | 96.5 ± 1.2 | 97.2 ± 0.8 | 1.26 (NS) | 9.67 (0.001) | 0.60 (NS) |
Results are shown as mean ± se. Statistical significance was set at P≤ 0.05. Significant F/P ANOVA values appear in boldface. ILI%, Proportion of ILIs in the range indicated relative to the total of all ILIs less than 1 sec.
Analysis of the licking microstructure data (detailed as follows) for the ORXA-induced increase in 0.1 m sucrose meal size indicated that the results generally replicated the findings of experiment 1.
There was a range of gustatory hedonic responses to the three taste solutions. The mean burst size and number of licks in the first minute increased significantly as sucrose concentrations were increased under aCSF conditions (Table 2). However, there was no significant effect of ORXA on these measures (Table 2), suggesting no influence on orosensory tastant evaluation.
As with the gustatory evaluation measures, measures reflecting caloric intensity of the tastants also varied across the test solutions under baseline conditions. Meal duration varied significantly across taste solution conditions (Fig. 4A), as did the number of bursts (Fig. 4B) and the average ingestion rate, with the fastest consumption rate expressed for 1 m sucrose (Table 2). After 4V ORXA, meal duration was prolonged by 66% for the 0.1 m sucrose solution (Fig. 4A). ORXA prolonged the meal principally by adding more bursts to the meal (Fig. 4B), because mean burst size, mean pause duration, and pause time as a percentage of meal duration measures were not increased (Table 2).
Figure 4.
ORXA (1 nm) infused to the 4V increased 0.1 m sucrose meal consumption through modification of measures associated with postingestive feedback inhibition. A, Mean (+se) meal duration (minutes) after aCSF (white bar) and ORXA (black bars) injections in 4V cannula-fitted rats (n = 16) ingesting water and 0.1 and 1 m sucrose solutions (ANOVA: concentration F(2,30) = 15.52, P < 0.001; drug F(1,15) = 11.22, P < 0.004; interaction F(2,30) = 3.33; *, P < 0.05. B, Mean (+ se) number of licking bursts for the same conditions as in A, ORXA significantly increased the number of bursts, primarily for 0.1 m sucrose (concentration F(2,30) = 10.24, P < 0.001; drug F(1,15) = 8.89, P = 0.009; interaction F(2,30) = 2.84, P = 0.07; 0.1 m sucrose comparison t(15) = −2.34; *, P = 0.03).
Experiment 3: 3V ORXA infusions in rats with AP/NST lesions or sham procedures
The lesion status could be not be verified in four rats due to difficulties with histological preparation; thus, the data for these rats were discarded, resulting in 17 APX and eight sham rats for which the data were analyzed. Figure 5 shows example photomicrographs depicting the range of lesions observed. Lesions ranged from considerable but incomplete destruction of the AP to complete ablation of the AP with collateral damage to the subjacent dorsal medial and commissural subnuclei of the NST, consistent with the range of damage reported in previous studies using this preparation (49,50,51).
Figure 5.
Example photomicrographs depicting the range of lesions observed (×40 magnification). A, A brain from a sham lesioned animal reveals intact AP, NST, DMX (X), and hypoglossal motor nucleus (XII). CC, Central canal. B, Image (same level as A) from the brain of a rat in the lesion group typical of those exhibiting the most minimal damage. Here, the AP was considerably but incompletely destroyed. C, Image (same level as A) from the brain of a rat in the lesion group typical of those exhibiting the most extensive damage. In this animal, the AP was completely ablated, with collateral damage to the subjacent dorsal medial and commissural subnuclei of the NST, particularly on the left side. The border of the AP/NST is suggested by the dashed line.
Consummatory feeding
Meal size for test solutions under control conditions was comparable across sham and APX groups (Fig. 6), and there was no significant between-subjects effect [F(1,23) = 0.46, not significant (NS)], and no tastant × group interaction [F(2,46) = 0.89, NS]. A t test also confirmed no significant difference in meal size between the 0.1 m sucrose aCSF conditions for the sham and APX groups [t(23) = 0.80, P = 0.43]. In addition, for both groups under aCSF conditions, the taste solutions gave rise to the anticipated differences within the meal: There were clear differences in meal size (Fig. 6) and meal duration across tastants (Fig. 7), and initial lick rate and mean burst size also significantly increased with increases in sucrose concentration (Tables 3 and 4).
Figure 6.
AP lesions abolished the hyperphagic response to 3V injection of ORXA (1 nm). A, Mean (+ se) meal size (milliliters) values after aCSF (white bar) and ORXA (black bars) injections in 3V cannula-fitted sham operated rats (n = 8) ingesting water and 0.1 and 1 m sucrose solutions. ORXA increased meal size overall [drug F(1,7) = 16.91; P < 0.005] and specifically for 0.1 m sucrose [t(7) = −2.61; *, P = 0.035]. B, Same conditions as A for 3V cannula-fitted AP/NST lesioned rats (APX; n = 17). There was no significant effect of drug [F(1,16) = 0.80, NS] and no significant interaction [F(2,32) = 0.70, NS], and the paired comparison for 0.1 m sucrose was not significant [t(16) = −0.86, NS].
Figure 7.
The increase in 0.1 m sucrose meal duration after 3V infusion of ORXA (1 nm) in sham operated rats was attenuated in rats with AP/NST lesions (APX). A, Mean (+ se) meal duration (minutes) values after aCSF (white bars) and ORXA (black bars) injections in 3V cannula-fitted sham operated rats (n = 8) ingesting water and 0.1 and 1 m sucrose solutions. Meal duration was specifically increased by ORXA for 0.1 m sucrose [ANOVA: concentration F(2,14) = 21.90, P < 0.001; drug F(1,7) = 4.70, P = 0.067; interaction F(2,14) = 7.14, P = 0.007; 0.1 m sucrose paired comparison t(7) = −2.52; *, P = 0.04]. B, Same conditions as A for 3V cannula-fitted APX rats (n = 17) [ANOVA: concentration F(2,32) = 9.78, P < 0.001; drug F(1,16) = 8.26, P = 0.01; interaction [F(2,32) = 1.20, NS; 0.1 m sucrose paired comparison t(7) = −1.67, NS].
Table 3.
Licking measures across tastant and ORXA conditions for rats in the sham lesion group
| Measure | Water | 0.1 m sucrose | 1 m sucrose | ANOVA
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| aCSF | 3V ORXA | aCSF | 3V ORXA | aCSF | 3V ORXA | Drug F(1,7) (P) | Concentration F(2,14) (P) | Interaction F(2,14) (P) | |
| 90-min intake (ml) | 2.15 ± 0.41 | 2.24 ± 0.85 | 13.28 ± 3.68 | 23.78 ± 3.02 | 9.77 ± 1.00 | 12.53 ± 2.15 | 25.07 (0.002) | 19.77 (0.001) | 7.19 (0.007) |
| Meals initiated | 1.25 ± 0.16 | 1.88 ± 0.40 | 2.00 ± 0.33 | 2.38 ± 0.32 | 2.00 ± 0.27 | 2.63 ± 0.32 | 6.76 (0.04) | 2.58 (0.02) | 0.06 (NS) |
| Meal lick count | 341 ± 88 | 235 ± 110 | 1948 ± 472 | 3479 ± 542 | 1268 ± 264 | 1645 ± 262 | 10.74 (0.01) | 25.10 (0.001) | 4.37 (0.03) |
| Latency (sec) | 154.80 ± 100.63 | 70.60 ± 23.21 | 18.12 ± 8.43 | 24.71 ± 13.04 | 8.70 ± 3.37 | 39.22 ± 15.21 | 0.19 (NS) | 2.97 (NS) | 0.91 (NS) |
| First minute lick rate | 86.38 ± 29.89 | 107.38 ± 46.02 | 210.13 ± 51.99 | 188.13 ± 42.48 | 213.13 ± 28.18 | 257.00 ± 26.56 | 0.22 (NS) | 8.43 (0.004) | 0.40 (NS) |
| Mean burst size (licks) | 39.13 ± 4.74 | 69.71 ± 22.16 | 95.57 ± 11.91 | 97.42 ± 10.46 | 94.50 ± 17.52 | 92.27 ± 15.02 | 0.51 (NS) | 5.64 (0.02) | 0.75 (NS) |
| Mean lick rate (licks/min) | 64.89 ± 21.16 | 213.88 ± 55.45 | 104.41 ± 18.36 | 72.32 ± 5.75 | 138.43 ± 22.55 | 91.82 ± 8.67 | 1.06 (NS) | 1.59 (NS) | 11.43 (0.001) |
| Burst count | 9.38 ± 2.98 | 3.38 ± 1.25 | 21.50 ± 5.51 | 41.00 ± 8.40 | 17.88 ± 7.51 | 21.75 ± 4.62 | 2.71 (NS) | 10.86 (0.001) | 2.45 (NS) |
| Pause time (%) | 91.41 ± 2.42 | 81.83 ± 6.31 | 76.32 ± 6.91 | 83.18 ± 1.87 | 66.64 ± 9.56 | 76.92 ± 1.83 | 0.21 (NS) | 8.23 (0.02) | 2.48 (NS) |
| Mean pause duration (sec) | 104.86 ± 31.03 | 75.78 ± 40.33 | 55.23 ± 17.49 | 78.29 ± 23.43 | 30.41 ± 12.06 | 62.74 ± 15.45 | 0.33 (NS) | 2.61 (NS) | 0.82 (NS) |
| Lick volume (μl) | 5.18 ± 0.49 | 5.65 ± 1.26 | 5.64 ± 0.46 | 5.14 ± 0.30 | 5.80 ± 0.29 | 5.59 ± 0.39 | 0.03 (NS) | 0.12 (NS) | 0.39 (NS) |
| Within-burst lick rate (licks/sec) | 6.0 ± 0.3 | 6.3 ± 0.4 | 6.5 ± 0.1 | 6.8 ± 0.1 | 6.6 ± 0.2 | 6.8 ± 0.2 | 0.94 (NS) | 5.83 (0.02) | 0.05 (NS) |
| Mean ILI (0–249 msec) | 148.19 ± 3.99 | 134.91 ± 4.19 | 142.61 ± 2.30 | 140.16 ± 2.80 | 142.67 ± 3.46 | 140.48 ± 2.85 | 9.19 (0.02) | 0.003 (NS) | 3.75 (0.05) |
| ILI% (0–249 msec) | 93.36 ± 2.03 | 94.39 ± 2.54 | 96.41 ± 0.95 | 97.70 ± 0.76 | 95.74 ± 1.87 | 98.07 ± 0.54 | 0.87 (NS) | 3.20 (0.07) | 0.22 (NS) |
Results are shown as mean ± se. Statistical significance was set at P ≤ 0.05. Significant F/P ANOVA values appear in boldface. ILI%, Proportion of ILIs in the range indicated relative to the total of all ILIs less than 1 sec.
Table 4.
Licking measures across tastant and ORXA conditions for rats in the APX lesion group
| Measure | Water | 0.1 m Sucrose | 1 m Sucrose | ANOVA
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| aCSF | 3V ORXA | aCSF | 3V ORXA | aCSF | 3V ORXA | Drug F(1,16) (P) | Concentration F(2,32) (P) | Interaction F(2,32) (P) | |
| 90-min intake (ml) | 0.83 ± 0.25 | 2.76 ± 1.10 | 18.26 ± 3.39 | 17.63 ± 3.86 | 14.00 ± 1.44 | 13.78 ± 1.83 | 0.08 (NS) | 20.10 (0.001) | 0.52 (NS) |
| Meals initiated | 1.24 ± 0.14 | 1.47 ± 0.21 | 1.94 ± 0.20 | 1.47 ± 0.19 | 1.71 ± 0.17 | 2.12 ± 0.17 | 0.13 (NS) | 4.60 (0.02) | 6.87 (0.003) |
| Meal lick count | 133 ± 52 | 495 ± 193 | 3366 ± 652 | 3944 ± 872 | 2504 ± 348 | 2419 ± 426 | 1.29 (NS) | 17.75 (0.001) | 0.73 (NS) |
| Latency (sec) | 78.90 ± 37.49 | 254.20 ± 129.20 | 77.78 ± 54.32 | 408.21 ± 391.12 | 7.67 ± 3.46 | 6.18 ± 4.62 | 2.34 (NS) | 0.77 (NS) | 0.63 (NS) |
| First minute lick rate | 19.54 ± 5.36 | 34.38 ± 13.94 | 152.31 ± 28.52 | 180.00 ± 31.21 | 308.46 ± 18.16 | 202.85 ± 23.57 | 2.24 (NS) | 63.07 (0.001) | 8.24 (0.002) |
| Mean burst size (licks) | 16.19 ± 6.20 | 22.76 ± 5.17 | 54.06 ± 10.33 | 74.16 ± 9.55 | 100.81 ± 14.47 | 89.73 ± 11.18 | 0.46 (NS) | 33.65 (0.001) | 1.81 (NS) |
| Mean lick rate (licks/min) | 107.35 ± 55.25 | 73.45 ± 25.44 | 107.74 ± 14.06 | 71.21 ± 12.35 | 195.14 ± 20.03 | 130.36 ± 18.67 | 2.92 (NS) | 4.71 (0.02) | 0.23 (NS) |
| Burst count | 8.31 ± 2.35 | 20.23 ± 5.16 | 67.23 ± 18.07 | 49.15 ± 10.62 | 33.85 ± 6.73 | 30.62 ± 4.80 | 0.26 (NS) | 8.97 (0.001) | 2.24 (NS) |
| Pause time (%) | 89.92 ± 2.33 | 86.54 ± 5.00 | 72.16 ± 4.46 | 79.84 ± 3.82 | 52.08 ± 5.46 | 63.00 ± 5.98 | 1.23 (NS) | 33.32 (0.001) | 1.90 (NS) |
| Mean pause duration (sec) | 46.01 ± 11.35 | 59.31 ± 10.86 | 28.74 ± 5.12 | 76.49 ± 33.05 | 20.85 ± 3.23 | 40.17 ± 10.35 | 4.43 (0.07) | 1.40 (NS) | 0.69 (NS) |
| Lick volume (μl) | 4.43 ± 0.63 | 4.83 ± 1.34 | 5.13 ± 0.61 | 5.56 ± 1.23 | 5.34 ± 0.43 | 5.13 ± 0.46 | 0.12 (NS) | 0.43 (NS) | 0.40 (NS) |
| Within-burst lick rate (licks/sec) | 4.2 ± 0.3 | 4.2 ± 0.3 | 5.5 ± 0.4 | 5.8 ± 0.4 | 6.2 ± 0.2 | 6.1 ± 0.2 | 0.03 (NS) | 9.82 (0.001) | 0.33 (NS) |
| Mean ILI (0–249 msec) | 147.55 ± 3.87 | 156.75 ± 3.67 | 151.29 ± 2.70 | 150.29 ± 3.24 | 149.78 ± 2.96 | 155.21 ± 4.90 | 5.39 (0.04) | 0.25 (NS) | 2.77 (NS) |
| ILI% (0–249 msec) | 75.79 ± 5.21 | 73.18 ± 7.33 | 90.30 ± 3.75 | 93.50 ± 2.46 | 96.77 ± 0.78 | 97.25 ± 0.82 | 0.04 (NS) | 10.38 (0.001) | 0.59 (NS) |
Results are shown as mean ± se. Statistical significance was set at P ≤ 0.05. Significant F/P ANOVA values appear in boldface. ILI%, Proportion of ILIs in the range indicated relative to the total of all ILIs less than 1 sec.
Sham group.
In the sham group, 3V ORXA effects on licking for sucrose were comparable to those observed after a 4V infusion (experiment 2). The 3V ORXA significantly increased meal size for the 0.1 m sucrose solution (Fig. 6A and Table 3).
The ORXA increase in 0.1 m sucrose meal size in the sham group was mediated by a doubling of meal duration (Fig. 7A). The mean burst count for 0.1 m sucrose was also nearly doubled after 3V ORXA, but the difference was not statistically significant (Table 3). There was no effect of ORXA on the initial lick rate or the mean burst size (Table 3). The results support the interpretation that 3V ORXA diminished inhibitory postingestive feedback but that it did not influence gustatory evaluation.
ORXA increased intake of intact rats to the same extent regardless of infusion site. After 3V or 4V ORXA, the net increases in meal size for 0.1 m sucrose were comparable to within 10% of each other. When the ORXA responses were compared (ORXA tastant × 3V/4V group), there was no significant main effect or group × drug interaction (all F values < 1.44, NS). The difference scores (ORXA minus aCSF) for each tastant were also not different [group: F(1,22) = 0.01, NS; tastant × group: F(2,44) = 0.36, NS].
APX group.
The AP/NST lesion did not disrupt responses to the tastants under baseline (aCSF) conditions. In the APX group, similar to the other experimental groups, meal sizes after 3V aCSF varied significantly across taste solutions (Fig. 6B), initial lick rate and mean burst size significantly increased with increases in sucrose concentration, and the meal duration, number of bursts in the meal, and pause times varied significantly across solutions (Table 4 and Figs. 6B and 7B).
Although more than twice as many lesioned rats were tested (n = 17) relative to the sham group (n = 8), 3V ORXA infusion produced no increases in meal size for any taste solution in the APX group. No measures of intake, including whole session intake, meal size, meal lick counts, or meal duration or burst count were significantly increased after 3V ORXA (Table 4 and Figs. 6B and 7B).
Appetitive feeding
In both the sham and APX groups, the number of meals initiated was significantly increased by 3V ORXA (Tables 3 and 4). In the sham group, the increase was expressed for all taste solutions, emphasizing an effect of ORXA on appetitive behaviors that was dissociated from its effect on meal size, which was increased only for 0.1 m sucrose. In the APX group, a statistically significant interaction indicated an effect of ORXA on meal frequency, but it was more difficult to interpret due to a reduction in the number of meals for 0.1 m sucrose and increases for the other two taste solutions (see Table 4). Nevertheless, this result was dissociated from the lack of ORXA effect on meal size for any tastant.
Experiment 4: responses to other stimuli in AP/NST lesioned rats
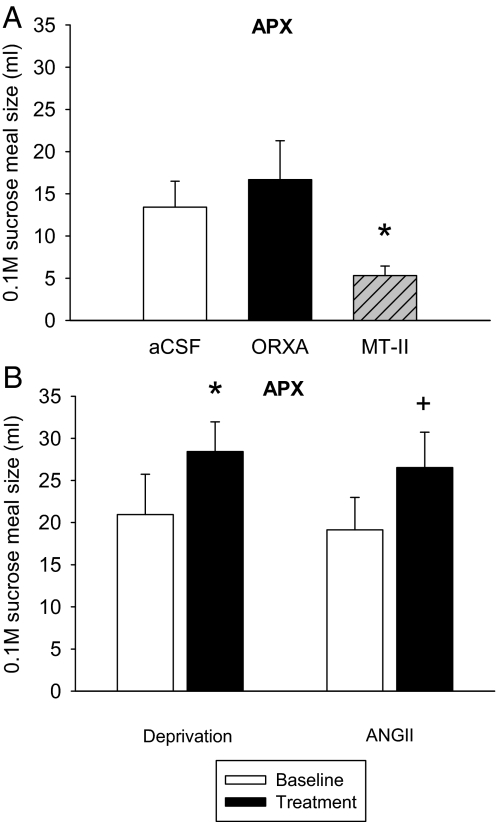
The subgroup of APX rats tested in experiment 3 that received a 3V MTII infusion after the main experiment (n = 10) exhibited a significant reduction of 0.1 m sucrose intake after infusion (Fig. 8A). Meal size responses in this subgroup also were not increased after ORXA infusion.
Figure 8.
A, Infusion of MTII (1 nm) significantly reduced meal size in AP/NST lesioned (APX) rats [t(9) = 2.46; P < 0.04], but ORXA (1 nm) infusions were without effect [t(9) = −0.85, NS]. Mean (+ se) meal size for the MTII-tested subgroup (n = 10) is shown. B, APX rats did increase mean (+ se) meal size for hypotonic 0.1 m sucrose after 22.5h food and fluid deprivation [t(9) = −2.75; *, P = 0.023], and they exhibited a marginally significant trend in the same direction after angiotensin II [ANGII; t(9) = −2.21; +, P = 0.055].
The second group of APX rats with confirmed lesions (n = 10) exhibited a significant increase in consumption of 0.1 m sucrose after 22.5 h food and water deprivation and a marginally significant increase after 3V injection of angiotensin II (Fig. 8B).
Discussion
As with previous studies, we found that ORXA injections to the 3V of intact rats significantly increased the consumption of water and sucrose solutions. We found similar results after 4V ORXA infusions. Our analysis of the behavioral processes underlying this hyperphagia (discussed below) lead us to conclude the following points: 1) ORXA influences both appetitive and consummatory feeding behaviors, but it does so through dissociable mechanisms; 2) ORXA increases consummatory feeding (meal size) through a diminution of postingestive inhibitory feedback, with no evident influence on gustatory evaluation; and 3) hindbrain structures may mediate the consummatory feeding responses to icv ORXA.
ORXA differentially influences appetitive and consummatory feeding
In experiment 1, the 4V 1 and 10 nm ORXA doses each increased 0.1 m sucrose intake, but the 1 nm dose increased only meal size (consummatory feeding), whereas the 10 nm dose increased meal frequency (appetitive feeding). In experiment 2, the 4V 1 nm ORXA dose increased meal size only for 0.1 m sucrose, whereas it increased meal frequency for all tastants. The same dissociation was observed in sham lesioned rats after 3V ORXA (1 nm). The results are consistent with a previous report that 3V ORXA (15) increased intraoral intake (a model of consummatory feeding (52) of 0.1 m sucrose. Finally, AP lesions abolished the increases in meal size after 3V ORXA, but increases in meal frequency were still observed.
The meal frequency results described here are consistent with the well known activating effects of ORXA (15), which has been reported to increase locomotor and exploratory activity (4), reward learning (7,12), feeding frequency (14), response to caloric deprivation, and willingness to work for food (6). ORXA neurons are moderately active during feeding and maximally active during exploratory behavior (53), and they may mediate fasting-induced waking processes. Collectively, these reports implicate ORXA in foraging behavior (see Ref. 4 for discussion), and our results are consistent with this interpretation. However, our results also indicate that ORXA separately influenced consummatory feeding through at least partially nonoverlapping sites of action relative to those for meal frequency effects.
ORXA suppresses inhibitory postingestive feedback (satiation)
We analyzed the licking microstructure of intact rats after 3V or 4V ORXA administration. Numerous studies show that under controlled laboratory conditions, licking patterns vary reliably with the orosensory (gustatory) and postingestive (satiating) stimulation properties of the tastant. Because very little is ingested early in the meal, orosensory evaluation of the tastant is the primary influence on behavior in this phase (41,42,43,44,54). The initial rate of licking in the meal varies systematically with increases in the concentration of palatable or aversive tastants (45,46,55). The mean lick burst size also increases or decreases in accordance with the palatability of the stimulus (32,45,46,55). As the meal progresses, the increasing size of the gastrointestinal load produces inhibitory feedback that leads to an increasingly rapid decline in ingestion rate. Increasing the caloric or volumetric properties of food produces more rapid reductions in lick rate, burst number, and meal duration (but not mean burst size or lick rate within bursts), suggesting that these measures reflect postingestive satiation (42,43,56,57,58,59,60). These response patterns were replicated across taste solutions in the control conditions of experiments 2 and 3.
ORXA did not affect initial lick rate or mean burst size in any experiment. This suggests that previous findings of ORXA increases in consumption of palatable foods such as sweetened chow or high-fat diet were not due to an ORXA influence on hedonic gustatory evaluation. This conclusion is also supported by reports that behavioral responses to the orexin 1 receptor antagonist SB-334867 did not overlap those to bitter quinine hydrochloride (61,62). Our results also suggest that previous reports of increases in water consumption (1,11) may be due to the action of ORXA to increase appetitive feeding (foraging) rather than a specific action on thirst-related systems (1,63), because neither meal size nor microstructure for water licking was affected by ORXA.
The current findings suggest that icv ORXA suppressed inhibitory postingestive feedback. After ORXA administration, rats prolonged the meal in all experiments as much as 2-fold, and this was achieved by increasing the number of licking bursts in the meal. Similar findings were reported by Ida et al. (10) who described increased total feeding duration after forebrain ORXA administration. Furthermore, a videographic analysis of feeding behavior after forebrain icv infusions of either ORXA or SB-334867 indicated that ORXA and the antagonist respectively delayed or hastened the onset of behavioral satiety (the transition from eating to resting) by 12–15 min, with no effects on locomotor behavior at low to moderate doses (8).
Our results are also consistent with studies that implicate ORXA in gastrointestinal processing. Orexin 1 receptors have been localized to neurons in the DMX that innervate the gastrointestinal tract (64), and ORXA stimulation enhances inhibitory post-synaptic currents in DMX neurons (65) and influences gastric acid and pancreatic secretions (3,66,67). ORXA applied to the DMX at the level of the AP was reported to increase gastric contractions (2). Intracerebroventricular ORXA has also been shown to alter gastric motility (9).
Finally, it is important to note that ORXA-induced increases in meal size were not due to nonspecific oromotor activation. Taste-related differences in response to the taste solutions were sustained after ORXA, consummatory responses to water were unaffected by ORXA, and ORXA did not increase the rate of licking within bursts.
Hindbrain structures may mediate the consummatory feeding responses to ORXA
We found that AP/NST lesions abolished meal size responses to 3V ORXA without influencing sensitivity to the gustatory and caloric properties of the test solutions or to the hyperphagic/dipsic effects of deprivation and angiotensin II, or the anorectic effects of MTII. The 3V and 4V ORXA effects on meal size were nearly identical in magnitude in intact rats, as was the qualitative pattern of the behavioral processes underlying meal size increases, with microstructure measures of postingestive feedback inhibition similarly affected in the 3V sham and the 4V groups. These results suggest that hindbrain sites involving the AP/NST may be important for consummatory feeding responses to icv ORXA stimulation.
It is worth noting that the AP/NST lesions did not eliminate appetitive (meal frequency) responses to 3V ORXA. Previous reports note that intraparenchymal infusions of low doses of ORXA to the paraventricular, dorsomedial, and perifornical/LH areas increase chow and/or water intake. These or other sites of ORXA action may mediate these intake increases through increases in appetitive feeding behaviors, independent of the neural systems that mediate ORXA consummatory feeding effects.
The precise brainstem sites of ORXA feeding action remain to be determined. Although there is evidence of ORXA ligand and orexin-1 receptors throughout structures of the dorsal vagal complex (see introductory section), previous studies showed that intra-NST ORXA infusions only minimally influenced feeding responses (11,31); interestingly, the extent of spread to the AP in those studies may have been limited (see Fig. 14F in Ref. 11). Our data suggest that the AP may be an important structure mediating consummatory feeding responses to icv ORXA, which could clarify the discrepancies between the reported 4V and intra-NST ORXA feeding responses. It is also possible that the resolution of our behavioral analysis, or our choice of foodstuffs, permitted us to observe significant ORXA responses that were not available to previous investigators, considering that we observed meal size effects only for the moderately palatable/caloric sucrose solution. The role of the AP in ORXA hyperphagia could be further clarified by analysis of 4V ORXA infusions in AP-lesioned rats and direct AP injections of orexin receptor ligands.
Finally, although we show that the AP/NST is necessary for consummatory ORXA responses, this does not prove that direct LH-AP ORXA projections are of central importance in mediating the ORXA consummatory feeding response. It is possible that second- and n-order mechanisms sensitive to ORXA stimulation convey ORXA-related signals to the hindbrain. However, our results do indicate that the AP/NST is an obligatory relay within any such ORXA sensitive networks.
Limitations
It is unclear why ORXA modified meal structure specifically for the 0.1 m sucrose solution without affecting the responses for 1 m sucrose. Relative to 1 m sucrose, the 0.1 m sucrose solution was both less palatable and less caloric. Because we observed no effect of ORXA on measures of gustatory evaluation (initial lick rate and mean burst size), the moderate caloric basis of the 0.1 m sucrose solution appears to be principal to the meal size effect of ORXA in this study. It is possible that the caloric density of 1 m sucrose produced stronger satiation signals that were not sufficiently subdued by our near-threshold dose of ORXA (a ceiling effect); however, others have reported that ORXA increased consumption after allowing animals to preingest food (12). Furudono et al. (9) reported that lateral ventricle ORXA infusions increased consumption of noncaloric 0.1% saccharin solution, but it remains unclear whether this effect (or the aforementioned) was due to appetitive responses to ORXA, which we observed for all solutions including water and 1 M sucrose. It would be worthwhile to evaluate whether ORXA influences consummatory responses for noncaloric or isopalatable solutions of different caloric density.
We also found that 4V ORXA dose-dependently increased the number of meals initiated (Fig. 1), a result we did not observe after 4V NPY infusions (32). However, our result is consistent with the reports that ghrelin and strong MTII doses influenced meal frequency after hindbrain delivery (69,70). Initially, these data appear at odds with the well known finding that chronic decerebrate rats, in which supracollicular structures are severed from the hindbrain, do not express appetitive feeding behaviors (including meal initiation), implying that hindbrain sites do not mediate appetitive functions (71). In attempting to reconcile these apparent contradictions, there are several possibilities to consider. It is possible that hindbrain ORXA stimulation in nonlesioned rats influenced appetitive feeding control systems in the forebrain through intact hindbrain-forebrain relays; there are, for example, direct ascending projections from the NST to the paraventricular hypothalamus and LH (72,73). Alternatively, it may be that appetitive behavioral responses are controlled via hindbrain structures, but these systems require permissive input from forebrain structures (74,75). Lastly, it is possible that there was back diffusion of the 4V ORXA injection to the forebrain ventricles (against the caudal flow of CSF), although several factors call this into question. First, previous studies have not observed ink flow to the forebrain after a 4V injection (25,32,40,41). Second, dipsogenic responses to angiotensin II are not elicited after 4V application. Third, 4V injections of MCH failed to influence feeding even when delivered at maximal doses (32). Regardless, our findings after APX indicate that the AP/NST is necessary for the expression of hyperphagic consummatory responses, even when ORXA is delivered to the 3V.
Comparisons with the behavioral effects of other hypothalamic peptides
Although MCH and ORXA have a common origin (the LH area) and common effects on food intake, the two peptides influence feeding behavior in profoundly different ways, and through different sites of action. The 3V MCH increased meal size by increasing measures of gustatory evaluation (mean burst size and initial lick rate), without influencing the postingestive feedback-related measures. ORXA produced the opposite pattern after either 3V or 4V infusions. In further contrast, MCH injection to the 4V was entirely without effect (32), suggesting that unlike ORXA and NPY, the hyperphagic effects of MCH require forebrain stimulation. This conclusion dovetails with other studies reporting that ORXA but not MCH feeding responses were blocked by the opioid antagonist naloxone (77), that subthreshold dose coinfusions of 3V ORXA/NPY and MCH did not synergistically increase food intake (78), and that forebrain icv NPY and ORXA modulated gastric motility, whereas forebrain icv MCH infusions did not under the same conditions (9).
Overall, ORXA actions were more similar to those of NPY and POMC receptor ligands. Like ORXA, NPY delivered to the 3V increased meal frequency for water, sucrose, and saccharin solutions, but meal size was increased only for caloric sucrose solutions (32,40). Furthermore, 3V and 4V NPY and ORXA, and 4V MTII infusions, affected licking measures associated with satiating postingestive feedback, not those associated with gustatory evaluation (32,40,41,76). In addition, subthreshold 3V NPY and ORXA doses did synergistically increase food intake (78).
Future studies exploring the effects of ORXA antagonists in the 4V or hindbrain nuclei, applied separately and conjointly with NPY or POMC receptor ligands, could provide additional support for the hypothesis that NPY, ORXA and POMC receptor ligands influence common mechanisms of meal size control through sites of action in the caudal brain stem. It is worth noting in this regard that the hindbrain mechanisms mediating responses to NPY, ORXA, and POMC are likely to be partially rather than completely overlapping, because rats with AP/NST lesions retained consummatory responses to forebrain-delivered MTII. Although it is true that icv administration of any compound can never truly mimic the quantitative, spatial, and temporal dynamics of the natural system, it is clear that the consummatory hyperphagia elicited here by forebrain icv ORXA stimulation required the integrity of the AP/NST. Overall, these findings further clarify the functional neuroanatomy of the central ORXA system, and they may shed further light on the organization of hypothalamic-hindbrain controls of feeding behavior.
Acknowledgments
We thank Shannon Fischer for assistance with the manuscript.
Footnotes
This work was supported by National Institutes of Health Grant DC07389, Howard Hughes Medical Institute, and Amherst College.
Disclosure Statement: The authors have nothing to disclose.
First Published Online November 13, 2008
Abbreviations: aCSF, Artificial cerebrospinal fluid; AP, area postrema; DMX, dorsal vagal motor nucleus; icv, intracerebroventricular; ILI, inter-lick interval; LH, lateral hypothalamic; MCH, melanin-concentrating hormone; MTII, melanotan II; NPY, neuropeptide Y; NS, not significant; ORXA, orexin-A; POMC, proopiomelanocortin; 3V, third ventricle; 4V, fourth ventricle.
References
- Kunii K, Yamanaka A, Nambu T, Matsuzaki I, Goto K, Sakurai T 1999 Orexins/hypocretins regulate drinking behaviour. Brain Res 842:256–261 [DOI] [PubMed] [Google Scholar]
- Krowicki ZK, Burmeister MA, Berthoud HR, Scullion RT, Fuchs K, Hornby PJ 2002 Orexins in rat dorsal motor nucleus of the vagus potently stimulate gastric motor function. Am J Physiol Gastrointest Liver Physiol 283:G465–G472 [DOI] [PubMed] [Google Scholar]
- Miyasaka K, Masuda M, Kanai S, Sato N, Kurosawa M, Funakoshi A 2002 Central orexin-A stimulates pancreatic exocrine secretion via the vagus. Pancreas 25:400–404 [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Ishii Y, Halford JC, Blundell JE 2002 Orexins and appetite regulation. Neuropeptides 36:303–325 [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M 1998 Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92:1, page following 696 [DOI] [PubMed] [Google Scholar]
- Thorpe AJ, Cleary JP, Levine AS, Kotz CM 2005 Centrally administered orexin A increases motivation for sweet pellets in rats. Psychopharmacology (Berl) 182:75–83 [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G 2005 A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437:556–559 [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Halford JC, Nunes de Souza RL, Canto de Souza AL, Piper DC, Arch JR, Blundell JE 2000 Dose-response effects of orexin-A on food intake and the behavioural satiety sequence in rats. Regul Pept 96:71–84 [DOI] [PubMed] [Google Scholar]
- Furudono Y, Ando C, Yamamoto C, Kobashi M, Yamamoto T 2006 Involvement of specific orexigenic neuropeptides in sweetener-induced overconsumption in rats. Behav Brain Res 175:241–248 [DOI] [PubMed] [Google Scholar]
- Ida T, Nakahara K, Katayama T, Murakami N, Nakazato M 1999 Effect of lateral cerebroventricular injection of the appetite-stimulating neuropeptide, orexin and neuropeptide Y, on the various behavioral activities of rats. Brain Res 821:526–529 [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud HR 2005 Orexin-A projections to the caudal medulla and orexin-induced c-Fos expression, food intake, and autonomic function. J Comp Neurol 485:127–142 [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud HR 2007 Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci 27:11075–11082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ER, Rada P, Darby RD, Leibowitz SF, Hoebel BG 2007 Orexigenic peptides and alcohol intake: differential effects of orexin, galanin, and ghrelin. Alcohol Clin Exp Res 31:1858–1865 [DOI] [PubMed] [Google Scholar]
- Haynes AC, Jackson B, Overend P, Buckingham RE, Wilson S, Tadayyon M, Arch JR 1999 Effects of single and chronic intracerebroventricular administration of the orexins on feeding in the rat. Peptides 20:1099–1105 [DOI] [PubMed] [Google Scholar]
- Benoit SC, Clegg DJ, Woods SC, Seeley RJ 2005 The role of previous exposure in the appetitive and consummatory effects of orexigenic neuropeptides. Peptides 26:751–757 [DOI] [PubMed] [Google Scholar]
- Backberg M, Hervieu G, Wilson S, Meister B 2002 Orexin receptor-1 (OX-R1) immunoreactivity in chemically identified neurons of the hypothalamus: focus on orexin targets involved in control of food and water intake. Eur J Neurosci 15:315–328 [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS 1998 Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18:9996–10015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE 1992 The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol 319:218–245 [DOI] [PubMed] [Google Scholar]
- Zamir N, Skofitsch G, Bannon MJ, Jacobowitz DM 1986 Melanin-concentrating hormone: unique peptide neuronal system in the rat brain and pituitary gland. Proc Natl Acad Sci USA 83:1528–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir N, Skofitsch G, Jacobowitz DM 1986 Distribution of immunoreactive melanin-concentrating hormone in the central nervous system of the rat. Brain Res 373:240–245 [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Air EL, Benoit SC, Sakai RS, Seeley RJ, Woods SC 2003 Intraventricular melanin-concentrating hormone stimulates water intake independent of food intake. Am J Physiol Regul Integr Comp Physiol 284:R494–R499 [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK 2002 The need to feed: homeostatic and hedonic control of eating. Neuron 36:199–211 [DOI] [PubMed] [Google Scholar]
- Sahu A 1998 Leptin decreases food intake induced by melanin-concentrating hormone (MCH), galanin (GAL) and neuropeptide Y (NPY) in the rat. Endocrinology 139:4739–4742 [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte Jr D, Seeley RJ, Baskin DG 2000 Central nervous system control of food intake. Nature 404:661–671 [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Morrison C, Banfield BW, Randall JA, Browning KN, Travagli RA, Berthoud HR 2005 Melanin concentrating hormone innervation of caudal brainstem areas involved in gastrointestinal functions and energy balance. Neuroscience 135:611–625 [DOI] [PubMed] [Google Scholar]
- Morton GJ, Schwartz MW 2001 The NPY/AgRP neuron and energy homeostasis. Int J Obes Relat Metab Disord 25(Suppl 5):S56–S62 [DOI] [PubMed] [Google Scholar]
- Guan JL, Saotome T, Wang QP, Funahashi H, Hori T, Tanaka S, Shioda S 2001 Orexinergic innervation of POMC-containing neurons in the rat arcuate nucleus. Neuroreport 12:547–551 [DOI] [PubMed] [Google Scholar]
- Powis JE, Bains JS, Ferguson AV 1998 Leptin depolarizes rat hypothalamic paraventricular nucleus neurons. Am J Physiol 274:R1468–R1472 [DOI] [PubMed] [Google Scholar]
- Jobst EE, Enriori PJ, Cowley MA 2004 The electrophysiology of feeding circuits. Trends Endocrinol Metab 15:488–499 [DOI] [PubMed] [Google Scholar]
- Muroya S, Funahashi H, Yamanaka A, Kohno D, Uramura K, Nambu T, Shibahara M, Kuramochi M, Takigawa M, Yanagisawa M, Sakurai T, Shioda S, Yada T 2004 Orexins (hypocretins) directly interact with neuropeptide Y, POMC and glucose-responsive neurons to regulate Ca2+ signaling in a reciprocal manner to leptin: orexigenic neuronal pathways in the mediobasal hypothalamus. Eur J Neurosci 19:1524–1534 [DOI] [PubMed] [Google Scholar]
- Dube MG, Kalra SP, Kalra PS 1999 Food intake elicited by central administration of orexins/hypocretins: identification of hypothalamic sites of action. Brain Res 842:473–477 [DOI] [PubMed] [Google Scholar]
- Baird JP, Rios C, Loveland JL, Beck J, Tran A, Mahoney CE 2008 Effects of hindbrain melanin-concentrating hormone and neuropeptide Y administration on licking for water, saccharin, and sucrose solutions. Am J Physiol Regul Integr Comp Physiol 294:R329–R343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM 1998 Distribution of orexin receptor mRNA in the rat brain. FEBS Lett 438:71–75 [DOI] [PubMed] [Google Scholar]
- Lu XY, Bagnol D, Burke S, Akil H, Watson SJ 2000 Differential distribution and regulation of OX1 and OX2 orexin/hypocretin receptor messenger RNA in the brain upon fasting. Horm Behav 37:335–344 [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK 2001 Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435:6–25 [DOI] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K 1999 Distribution of orexin neurons in the adult rat brain. Brain Res 827:243–260 [DOI] [PubMed] [Google Scholar]
- Rogers RC, McCann MJ 1993 Intramedullary connections of the gastric region in the solitary nucleus: a biocytin histochemical tracing study in the rat. J Auton Nerv Syst 42:119–130 [DOI] [PubMed] [Google Scholar]
- Yang B, Ferguson AV 2002 Orexin-A depolarizes dissociated rat area postrema neurons through activation of a nonselective cationic conductance. J Neurosci 22:6303–6308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter RC, Slusser PG, Stone S 1981 Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science 213:451–452 [DOI] [PubMed] [Google Scholar]
- Baird JP, Gray NE, Fischer SG 2006 Effects of neuropeptide Y on feeding microstructure: dissociation of appetitive and consummatory actions. Behav Neurosci 120:937–951 [DOI] [PubMed] [Google Scholar]
- Baird JP, Rios C, Gray NE, Walsh CE, Fischer SG, Pecora AL 2006 Effects of melanin-concentrating hormone on licking microstructure and brief-access taste responses. Am J Physiol Regul Integr Comp Physiol 291:R1265–R1274 [DOI] [PubMed] [Google Scholar]
- Davis JD, Levine MW 1977 A model for the control of ingestion. Psychol Rev 84:379–412 [PubMed] [Google Scholar]
- Davis JD 1998 A model for the control of ingestion: 20 years later. Prog Psychobiol Physiol Psychol 17:127–173 [Google Scholar]
- Spector AC, Klumpp PA, Kaplan JM 1998 Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behav Neurosci 112:678–694 [DOI] [PubMed] [Google Scholar]
- Spector AC, St John SJ 1998 Role of taste in the microstructure of quinine ingestion by rats. Am J Physiol 274:R1687–R1703 [DOI] [PubMed] [Google Scholar]
- Baird JP, St John SJ, Nguyen EA 2005 Temporal and qualitative dynamics of conditioned taste aversion processing: combined generalization testing and licking microstructure analysis. Behav Neurosci 119:983–1003 [DOI] [PubMed] [Google Scholar]
- Kaplan JM, Baird JP, Grill HJ 2001 Dissociation of licking and volume intake controls in rats ingesting glucose and maltodextrin. Behav Neurosci 115:188–195 [DOI] [PubMed] [Google Scholar]
- Davis JD 1996 Deterministic and probabilistic control of the behavior of rats ingesting liquid diets. Am J Physiol 270:R793–R800 [DOI] [PubMed] [Google Scholar]
- Edwards GL, Ritter RC 1981 Ablation of the area postrema causes exaggerated consumption of preferred foods in the rat. Brain Res 216:265–276 [DOI] [PubMed] [Google Scholar]
- Edmonds BK, Edwards GL 1998 Dorsomedial hindbrain participation in glucoprivic feeding response to 2DG but not 2DG-induced hyperglycemia or activation of the HPA axis. Brain Res 801:21–28 [DOI] [PubMed] [Google Scholar]
- Edwards GL, Ritter RC 1986 Area postrema lesions: cause of overingestion is not altered visceral nerve function. Am J Physiol 251:R575–R581 [DOI] [PubMed] [Google Scholar]
- Flynn FW, Grill HJ 1988 Intraoral intake and taste reactivity responses elicited by sucrose and sodium chloride in chronic decerebrate rats. Behav Neurosci 102:934–941 [DOI] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM 2005 Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron 46:787–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendinning JI, Chyou S, Lin I, Onishi M, Patel P, Zheng KH 2005 Initial licking responses of mice to sweeteners: effects of tas1r3 polymorphisms. Chem Senses 30:601–614 [DOI] [PubMed] [Google Scholar]
- Hsiao S, Fan RJ 1993 Additivity of taste-specific effects of sucrose and quinine: microstructural analysis of ingestive behavior in rats. Behav Neurosci 107:317–326 [DOI] [PubMed] [Google Scholar]
- Davis JD, Smith GP, Kung TM 1994 Abdominal vagotomy alters the structure of the ingestive behavior of rats ingesting liquid diets. Behav Neurosci 108:767–779 [PubMed] [Google Scholar]
- Eisen S, Davis JD, Rauhofer E, Smith GP 2001 Gastric negative feedback produced by volume and nutrient during a meal in rats. Am J Physiol Regul Integr Comp Physiol 281:R1201–R1214 [DOI] [PubMed] [Google Scholar]
- Davis JD, Smith GP, Sayler JL 1997 Reduction of intake in the rat due to gastric filling. Am J Physiol 272:R1599–R1605 [DOI] [PubMed] [Google Scholar]
- Davis JD, Smith GP, Kung TM 1995 Abdominal vagotomy attenuates the inhibiting effect of mannitol on the ingestive behavior of rats. Behav Neurosci 109:161–167 [PubMed] [Google Scholar]
- Schwartz GJ, Salorio CF, Skoglund C, Moran TH 1999 Gut vagal afferent lesions increase meal size but do not block gastric preload-induced feeding suppression. Am J Physiol 276:R1623–R1629 [DOI] [PubMed] [Google Scholar]
- Ishii Y, Blundell JE, Halford JC, Upton N, Porter R, Johns A, Rodgers RJ 2004 Differential effects of the selective orexin-1 receptor antagonist SB-334867 and lithium chloride on the behavioural satiety sequence in rats. Physiol Behav 81:129–140 [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Halford JC, Nunes de Souza RL, Canto de Souza AL, Piper DC, Arch JR, Upton N, Porter RA, Johns A, Blundell JE 2001 SB-334867, a selective orexin-1 receptor antagonist, enhances behavioural satiety and blocks the hyperphagic effect of orexin-A in rats. Eur J Neurosci 13:1444–1452 [DOI] [PubMed] [Google Scholar]
- Ono K, Kai A, Honda E, Inenaga K 2008 Hypocretin-1/orexin-A activates subfornical organ neurons of rats. Neuroreport 19:69–73 [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Patterson LM, Sutton GM, Morrison C, Zheng H 2005 Orexin inputs to caudal raphe neurons involved in thermal, cardiovascular, and gastrointestinal regulation. Histochem Cell Biol 123:147–156 [DOI] [PubMed] [Google Scholar]
- Davis SF, Williams KW, Xu W, Glatzer NR, Smith BN 2003 Selective enhancement of synaptic inhibition by hypocretin (orexin) in rat vagal motor neurons: implications for autonomic regulation. J Neurosci 23:3844–3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Takahashi N, Tanno S, Nagamine M, Takakusaki K, Okumura T 2005 A selective orexin-1 receptor antagonist, SB334867, blocks 2-DG-induced gastric acid secretion in rats. Neurosci Lett 376:137–142 [DOI] [PubMed] [Google Scholar]
- Takahashi N, Okumura T, Yamada H, Kohgo Y 1999 Stimulation of gastric acid secretion by centrally administered orexin-A in conscious rats. Biochem Biophys Res Commun 254:623–627 [DOI] [PubMed] [Google Scholar]
- Guan JL, Wang QP, Kageyama H, Kita T, Takenoya F, Hori T, Shioda S 2005 Characterization of orexin A immunoreactivity in the rat area postrema. Regul Pept 129:17–23 [DOI] [PubMed] [Google Scholar]
- Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ 2003 Hyperphagic effects of brainstem ghrelin administration. Diabetes 52:2260–2265 [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Phifer CB, Berthoud HR 2005 Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections. Am J Physiol Regul Integr Comp Physiol 289:R247–R258 [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R 1978 Neurological tests and behavioral deficits in chronic thalamic and chronic decerebrate rats. Brain Res 143:299–312 [DOI] [PubMed] [Google Scholar]
- Nosaka S 1984 Solitary nucleus neurons transmitting vagal visceral input to the forebrain via a direct pathway in rats. Exp Neurol 85:493–505 [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW 1982 The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res 257:275–325 [DOI] [PubMed] [Google Scholar]
- Grill HJ, Kaplan JM 2002 The neuroanatomical axis for control of energy balance. Front Neuroendocrinol 23:2–40 [DOI] [PubMed] [Google Scholar]
- von Monakow C 1914 Die Lokalisation in Grosshirnrinde und der Abbau der Function durch Korticale herde. Wiesbaden, Germany: J.F. Bergmann [Google Scholar]
- Williams DL, Grill HJ, Weiss SM, Baird JP, Kaplan JM 2002 Behavioral processes underlying the intake suppressive effects of melanocortin 3/4 receptor activation in the rat. Psychopharmacology (Berl) 161:47–53 [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Air EL, Woods SC, Seeley RJ 2002 Eating elicited by orexin-A, but not melanin-concentrating hormone, is opioid mediated. Endocrinology 143:2995–3000 [DOI] [PubMed] [Google Scholar]
- Sahu A 2002 Interactions of neuropeptide Y, hypocretin-I (orexin A) and melanin-concentrating hormone on feeding in rats. Brain Res 944:232–238 [DOI] [PubMed] [Google Scholar]