Abstract
Background:
Extrapyramidal motor symptoms precede dementia in Parkinson disease (PDD) by many years, whereas dementia occurs early in dementia with Lewy bodies (DLB). Despite this clinical distinction, the neuropsychological and neuropathologic features of these conditions overlap. In addition to widespread distribution of Lewy bodies, both diseases have variable burdens of neuritic plaques and neurofibrillary tangles characteristic of Alzheimer disease (AD).
Objectives:
To determine whether amyloid deposition, as assessed by PET imaging with the β-amyloid–binding compound Pittsburgh Compound B (PiB), can distinguish DLB from PDD, and to assess whether regional patterns of amyloid deposition correlate with specific motor or cognitive features.
Methods:
Eight DLB, 7 PDD, 11 Parkinson disease (PD), 15 AD, and 37 normal control (NC) subjects underwent PiB-PET imaging and neuropsychological assessment. Amyloid burden was quantified using the PiB distribution volume ratio.
Results:
Cortical amyloid burden was higher in the DLB group than in the PDD group, comparable to the AD group. Amyloid deposition in the PDD group was low, comparable to the PD and NC groups. Relative to global cortical retention, occipital PiB retention was lower in the AD group than in the other groups. For the DLB, PDD, and PD groups, amyloid deposition in the parietal (lateral and precuneus)/posterior cingulate region was related to visuospatial impairment. Striatal PiB retention in the DLB and PDD groups was associated with less impaired motor function.
Conclusions:
Global cortical amyloid burden is high in dementia with Lewy bodies (DLB) but low in Parkinson disease dementia. These data suggest that β-amyloid may contribute selectively to the cognitive impairment of DLB and may contribute to the timing of dementia relative to the motor signs of parkinsonism.
GLOSSARY
- AAL
= Automated Anatomic Labeling;
- AD
= Alzheimer disease;
- ADRC
= Alzheimer’s Disease Research Center;
- AMNART
= American version of the National Adult Reading Test;
- ANCOVA
= analysis of covariance;
- BDS
= Blessed Dementia Scale;
- CAA
= cerebral amyloid angiopathy;
- CDR
= Clinical Dementia Rating;
- CDR-SB
= Clinical Dementia Rating Sum of Boxes;
- DLB
= dementia with Lewy bodies;
- DVR
= distribution volume ratio;
- FCSRT
= Cued Selective Reminding Test;
- FRSRT
= Free Selective Reminding Test;
- H&Y
= Hoehn and Yahr;
- MGH
= Massachusetts General Hospital;
- MMSE
= Mini-Mental State Examination;
- NC
= normal control;
- NFT
= neurofibrillary tangle;
- NPIQ
= Neuropsychiatric Inventory Questionnaire;
- NS
= not significant;
- PD
= Parkinson disease;
- PDD
= Parkinson disease dementia;
- PiB
= Pittsburgh Compound B;
- ROI
= region of interest;
- SPM2
= Statistical Parametric Mapping;
- UKPDSBRC
= UK Parkinson’s Disease Society Brain Bank Research Center;
- UPDRS
= United Parkinson’s Disease Rating Scale;
- WAIS-R
= Wechsler Adult Intelligence Scale–Revised.
Parkinson disease (PD) and dementia with Lewy bodies (DLB) are the most common synucleinopathies associated with dementia. A diagnosis of PD dementia (PDD) is made when cognitive impairments develop in the setting of well-established idiopathic PD,1 whereas in DLB, dementia often heralds the onset of illness in advance of parkinsonian motor signs but by consensus may follow their development up to 1 year from their onset.2
The clinical features of both DLB and PDD include hallucinations, cognitive fluctuations, and dementia in the setting of parkinsonism.1,2 The cognitive domains and broad neuropsychologic features that are impacted in PDD and DLB overlap substantially, with prominent executive dysfunction and visuospatial abnormalities.3 Despite the different temporal sequences of motor and cognitive deficits, at autopsy PDD and DLB show remarkably convergent neuropathologic changes. These include widespread limbic and cortical Lewy bodies4 as well as loss of cholinergic neurons in ventral forebrain nuclei.5 Neuritic plaques that contain amyloid and neurofibrillary tangles (NFTs) are found in the majority of cases of DLB4 and are common in PD.6 The overlap of clinical, neuropsychologic, and neuropathologic features has led to the hypothesis that PDD and DLB may be different phenotypic expressions of the same underlying process.7,8 However, it is possible that the neuropathologic bases for these two diseases overlap at autopsy because of their convergence late in their course. The capacity to measure amyloid burden antemortem with Pittsburgh Compound B (PiB) PET imaging permits us to examine whether differential amyloid burden correlates with the different natural histories of PDD and DLB. Specifically, we hypothesized that DLB and PDD could be differentiated by their degree of cortical amyloid burden. We further hypothesized that regional deposition of amyloid would correlate with cognitive and motor features of the DLB and PDD cohorts and would distinguish them from Alzheimer disease (AD), PD, and normal control (NC) subjects.
METHODS
Study design.
Eight subjects with DLB, 7 with PDD, and 11 with PD were recruited from the Massachusetts General Hospital (MGH) Movement and Memory Units for the study (table 1). All subjects with PD fulfilled criteria for a diagnosis of idiopathic PD according to the UK Parkinson’s Disease Society Brain Bank Research Center (UKPDSBRC) clinical diagnostic criteria,9 had Hoehn and Yahr (H&Y) stages I through IV, and were cognitively normal, with Blessed Dementia Scale (BDS) scores < 5. Subjects with PDD met UKPDSBRC criteria for idiopathic PD, were H&Y stages I through IV and, in addition, met Diagnostic and Statistical Manual of Mental Disorders, 3rd Edition, Revised, criteria for dementia, with BDS scores ≥ 5. Although the recent consensus criteria for PDD1 were unavailable at the study’s inception, all subjects with PDD would have met these criteria at the time of enrollment. Subjects with DLB met consensus criteria for probable DLB of the Dementia with Lewy Bodies Consortium,2 with the presence of at least two of the following: parkinsonism, hallucinations, and fluctuations in cognition. The BDS scores in DLB were ≥5. Six of 7 DLB, 11 of 11 PD, and 6 of 7 PDD subjects were treated with dopaminergic drugs, such as l-dopa. Acquired data from these parkinsonian subjects were compared with those of a separately collected cohort of 15 AD and 37 NC subjects who were participants in longitudinal studies of normal aging and dementia at MGH and Brigham and Women’s Hospital. The diagnosis of NC required normal neurologic examination results, a Clinical Dementia Rating (CDR) scale10 score of 0, and normal cognition, with a BDS score < 3 or Mini-Mental State Examination (MMSE) score > 27. All subjects with AD met National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association criteria for probable AD11 and had BDS scores ≥ 5 or MMSE scores < 23. All participants gave written informed consent according to the protocols approved by the Partners Healthcare Inc. Institutional Review Board.
Table 1 Demographic and clinical features

Clinical evaluation.
Clinical evaluation of motor function involved the H&Y staging of PD and United Parkinson’s Disease Rating Scale (UPDRS) part III12 testing in the on state; the mean lag from dose of dopaminergic drug to examination in 21 of 23 DLB, PDD, and PD subjects was not different between groups (p > 0.37). Additional measures included the CDR, the CDR Sum of Boxes, the Mayo fluctuations screen,13 the Neuropsychiatric Inventory Questionnaire,14 including the hallucinations subscale, and the Geriatric Depression Scale. Neuropsychological testing included the MMSE, BDS, digits forward, digits backward, Trails A and B, Logical Memory IA and IIA, Free and Cued Selective Reminding Tests (FRSRT and FCSRT, respectively),15 Benton visual form discrimination test, Wechsler Adult Intelligence Scale–Revised, letter fluency, two-item category fluency (animals and vegetables), 30-item Boston Naming Test, and the American version of the National Adult Reading Test verbal IQ assessment. The majority of these tests correspond with the Uniform Data Set of cognitive tests, as developed by the AD Centers. The mean interval between neuropsychological testing and PiB-PET was 0.07 ± 2.12 months.
PET imaging acquisition.
N-Methyl-[11C]2-(4-methylaminophenyl)-6-hydroxybenzothiazole (PiB) was prepared at Massachusetts General Hospital as described previously.16 Subjects were positioned in either of two PET cameras for dynamic acquisition, a Siemens/CTI ECAT HR scanner (three-dimensional mode; 63 image planes; 15.2-cm axial field of view; 5.6-mm transaxial resolution; 2.4-mm slice interval; 69 frames: 12 × 15 seconds, 57 × 60 seconds; Knoxville, TN) or a GE PC4096 scanner (two-dimensional mode; 15 image planes; 10.0-cm axial field of view; 7.0-mm transaxial resolution; 6.0-mm slice interval; 39 frames: 8 × 15 seconds, 4 × 60 seconds, 27 × 120 seconds; Milwaukee, WI). After a transmission scan, 8.5 to 15 mCi 11C-PiB was injected as a bolus and followed immediately by a 60-minute dynamic acquisition. PET data were reconstructed with ordered set expectation maximization and corrected for attenuation, and each frame was evaluated to verify adequate count statistics and absence of head motion.
PET image analysis.
Global and regional cortical PiB retention was calculated using the Logan graphical analysis method,17,18 with cerebellar cortex as the reference tissue input function, to evaluate specific PiB retention expressed as the distribution volume ratio (DVR) as in previous PiB studies.19–22 Parametric images of DVR over the late (40- to 60-minute) epoch were assessed visually and classified as PiB negative and positive without knowledge of clinical classification, by identifying the presence of either specific cortical PiB retention (at least 50 cortical voxels with DVR >1.3; PiB positive) or the presence of only nonspecific white matter uptake (much less than 50 cortical voxels with DVR >1.3; PiB negative).19,21 Subjects were classified as PiB positive if even a relatively restricted portion of cortex had evidence of specific binding; hence, aggregate binding in such individuals averaged over large areas of nonspecific binding remained low.
To compare PiB retention in regions of interest (ROIs) among groups, we transformed each subject’s PiB-PET data set into a standard space and calculated PiB DVR in anatomically defined ROIs, as described previously.22 The standard space used was the single-subject Automated Anatomic Labeling (AAL),23 and we chose Statistical Parametric Mapping (SPM2)24 to determine the warping transformation that carried each subject’s PiB-PET data into AAL space. The resulting warping transformation was applied to the individual dynamic frames of PiB data. Activity was measured in each individual PiB data set in the following ROI aggregates: global, occipital, frontal lateral, anterior cingulate, lateral temporal, medial temporal, parietal (lateral and precuneus)/posterior cingulate, and striatum. The components of each aggregate are tabulated in appendix e-1 on the Neurology® Web site at www.neurology.org. Time–activity curves were derived in each ROI aggregate in each subject, and the DVR was calculated according to standard methods,17,18 using the cerebellar gray as reference. We used analysis of covariance (ANCOVA) and ratios to derive regional retention in the ROI aggregates relative to global retention. We tested the following hypotheses relating regionally specific amyloid burden to cognitive test performance: parietal (lateral and precuneus)/posterior cingulate DVR and performance on the Benton visual form discrimination test; occipital DVR and performance on the Benton visual form discrimination test; frontal lateral DVR and performance on digits backward; frontal lateral DVR and performance on FCSRT; anterior cingulate DVR and performance on digits backward; anterior cingulate DVR and performance on FCSRT; medial temporal DVR and performance on FRSRT; occipital DVR and hallucinations. In a separate study, using overlapping subject groups, intercamera reliability was found to be satisfactory. The mean DVR in the global ROI did not differ between the two PET cameras among groups with or without dementia (age and multiple test adjusted tests, p > 0.30).
RESULTS
Clinical features.
Demographics of the cohorts are shown in table 1. MMSE scores were comparable between the DLB and PDD groups but were lower in the DLB group than in the AD group (p < 0.017, analysis of variance). The DLB and PDD groups had similar H&Y scores. However, the H&Y score was lower in the PD group than in the PDD group (p < 0.009). The DLB and PDD groups had more severe motor dysfunction than did the PD group as measured by the UPDRS III (DLB vs PD, p < 0.03; PDD vs PD, p < 0.006). The DLB and PDD groups performed similarly on the battery of cognitive tests. They had more cognitive fluctuations than did the PD group (DLB vs PD, p = 0.004; PDD vs PD, p = 0.008) and marginally more hallucinations than did the PD group (DLB vs PD, p = 0.055; PDD vs PD, p = 0.063). All NC subjects scored normally on all neuropsychologic tests.
Global amyloid burden.
Cortical amyloid burden differed among the AD, DLB, PDD, PD, and NC groups (p < 0.0001, nonparametric permutation test; figures 1 and 2). The DLB group had greater mean PiB retention than did the PDD group (p < 0.05, Tukey adjusted post hoc test), despite similar degrees of cognitive impairment (table 1). This difference remained significant after adjusting for nonsignificant differences in performance on the MMSE or BDS, with ANCOVA. The mean amyloid burden in the DLB group was comparable to levels associated with AD (difference not significant [NS]). PiB retention was higher in the DLB group than in the PD or NC group (p < 0.05). In the PDD group, PiB retention was lower than in the AD group (p < 0.05); the mean amyloid burden in the PDD group was not statistically different from that in the PD or NC group. Cortical amyloid burden in the PD group was similar to that in the NC group (NS). These findings persisted after adjusting for age with ANCOVA. There was neither a significant interaction of gender with diagnosis nor a gender main effect on amyloid burden (factorial ANCOVA with crossed diagnosis and gender factors and an age covariate).
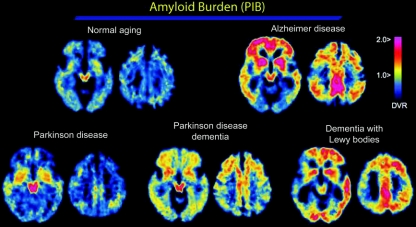
Figure 1 Representative PiB images
Images from a 75-year-old normal control (NC) (upper left), a 79-year-old patient with Alzheimer disease (AD) (Mini-Mental State Examination [MMSE] score 25; upper right), a 65-year-old patient with Parkinson disease (PD) (MMSE score 27; lower left), a 69-year-old patient with PD dementia (PDD) (MMSE score 25; lower middle), and a 71-year-old patient with dementia with Lewy bodies (DLB) (MMSE score 8; lower right) are displayed. Note that Pittsburgh Compound B (PiB) retention is qualitatively increased in AD, PDD, and DLB compared with NC and PD. Note also the variation in the regional distribution of amyloid across the AD, PDD, and DLB images. DVR = distribution volume ratio.

Figure 2 Amyloid deposition in Lewy body diseases
Global Pittsburgh Compound B (PiB) distribution volume ratio for Alzheimer disease (AD), dementia with Lewy bodies (DLB), Parkinson disease dementia (PDD), Parkinson disease (PD), and normal control (NC) groups. Each point represents a single subject. Cases with specific cortical uptake of PiB (i.e., “PiB positive”) are shown as closed circles; some subjects with small foci of PiB retention had low global averages. Cases with low, nonspecific binding are shown as open circles (i.e., “PiB negative”). Lines connect group means that do not significantly differ: the AD and DLB group means were not significantly different from each other, and the PD, PDD, and NC means were not significantly different from each other. All pairwise comparisons between these two clusters were significant (p ≤ 0.05, Tukey post hoc tests).
We visually assessed the parametric images to differentiate cases with specific cortical PiB retention (PiB positive) from cases with only nonspecific white matter uptake (PiB negative). All 15 AD subjects, 7 of 8 DLB subjects, and all 7 PDD subjects were PiB positive. Seven of 11 PD subjects and 19 of 37 NC subjects were PiB positive. PiB-positive and PiB-negative PD subjects overlapped in demographic, clinical, and neuropsychological features, and there were no significant differences in mean age, performance on the MMSE, or Hoehn and Yahr stage. PiB-positive and PiB-negative NC subjects similarly overlapped in demographic, clinical, and neuropsychological features, including age and MMSE performance.
Regional amyloid burden.
The specific PiB retention measured in ROIs reflected global PiB retention (absolute, unadjusted DVRs are shown in table 2). We therefore also analyzed differences between diagnostic groups in regional PiB retention, adjusting for global PiB retention via ANCOVA. Adjusted amyloid burden in the frontal lateral, lateral temporal, medial temporal, parietal (lateral and precuneus)/posterior cingulate, and anterior cingulate ROIs did not differentiate the DLB, PDD, or PD groups from the AD group (for all comparisons, NS). In contrast, adjusted occipital PiB retention was lower in the AD group than in the DLB (p = 0.013, Tukey adjusted post hoc test), PDD (p = 0.0002), or PD (p = 0.0004) group. The AD group had lower adjusted occipital PiB than the NC group as well (p < 0.0001). Adjusted PiB burden in the ROIs did not differentiate the DLB, PDD, and PD groups from one another.
Table 2 Regional PiB retention

Association of global amyloid with clinical variables in Lewy body diseases.
Across the DLB, PDD, and PD groups pooled, the greater the amyloid burden was, as measured by the global cortical PiB DVR, the lower the MMSE score was (r = −0.50, p = 0.01). However, this effect was dominated by differences between groups rather than by relations within groups, such that the pooled within-group relation was not significant (within group partial r = −0.1, p = 0.65).
Association of regional amyloid with clinical, cognitive, and motor variables in Lewy body diseases.
We tested eight hypotheses relating regionally specific amyloid burden with specific tests of cognitive performance across the DLB, PDD, and PD groups. Regional PiB retention was adjusted for global retention. We found that regional amyloid burden in the parietal (lateral and precuneus)/posterior cingulate region—but not in the occipital region—showed a within-group relation to decline in the Benton visual form discrimination test (whether we adjusted for global PiB retention as a simple ratio [p = 0.011; false discovery rate p = 0.047 correcting for multiple ROI–cognitive test comparisons] or via ANCOVA [p = 0.005]). In contrast, in AD and NC subjects, amyloid burden in this region did not correlate with Benton performance. Regional amyloid burden in the DLB, PDD, and PD groups did not relate significantly to the other hypothesized focal cognitive impairments, whether blind to diagnostic group or within diagnostic group. In particular, frontal executive tasks and cued recall (digits backward and FCSRT) did not relate to PiB retention in the frontal lateral or anterior cingulate regions; memory impairment (FRSRT) was not associated with increased PiB retention in the medial temporal lobe region; and hallucinations were not associated with occipital PiB retention.
There was a significant interaction of diagnostic group (DLB, PDD, PD) with the relation of striatal PiB retention to UPDRS III score (whether we adjusted for global PiB retention as a simple ratio [p = 0.039] or via ANCOVA [p = 0.046]). In the case of the DLB and PDD groups but not the PD group, the greater the amyloid burden in the striatum was, the lower (better) the UPDRS III score was (DLB, r = −0.87 p = 0.01; PDD, r = −0.90 p = 0.005).
DISCUSSION
We found that global cortical amyloid burden, as measured with PiB-PET, was higher in the DLB group than in the PDD group; in fact, PiB retention in the DLB group was comparable to retention in the AD group. In contrast to the DLB group, levels of amyloid burden in the PDD group were low, comparable to those of PD and NC subjects. The differential amyloid load of the DLB and PDD groups was not due to differences in dementia severity, which was comparable between the groups. These data demonstrate that despite their similar and overlapping clinical, neuropsychologic, and neuropathologic features, DLB and PDD can be differentiated on the basis of amyloid deposition. These findings confirm and extend prior reports regarding PiB imaging in parkinsonian syndromes. Prominent PiB uptake in DLB was noted in one small series25 and was confirmed in a single postmortem case.26 In another report, there was minimal cortical PiB binding in 10 PDD cases.27 In DLB, amyloid deposition has been shown to vary with the rate of cognitive decline and the development of clinical diagnostic features.25 This observation may extend to PDD as well, with the generalization that amyloid deposition reduces the duration between onset of clinical (motor or cognitive) symptoms and the onset of dementia. Based on our data, we postulate that when amyloid accumulates in the cerebral blood vessels and cortical parenchyma early in the course of parkinsonism, the clinical features of DLB are expressed. When amyloid deposition in the cortex is modest and a late event, the diagnosis of PDD is usually made.
Cortical PiB retention has been reported to occur in greater than 20% of apparently normal people,22,28 consistent with the prevalence of AD changes in the elderly without dementia.29,30 Our sample of NC subjects contained a larger fraction (51%) of PiB positives than previously reported. Whether this finding is related to higher cognitive reserve in our subjects31 and whether these deposits are harbingers of dementia remain to be established and are under intense investigation. In that context, it is noteworthy that while quantitatively modest, specific cortical PiB retention was present in many but not all cognitively normal PD subjects and in all cases of PDD. The cortical deposition of amyloid in PD may therefore be a risk factor for dementia. Longitudinal PiB-PET imaging of a PD cohort will be necessary to resolve this question.
DLB is associated with both neuritic dense core plaques and diffuse plaques,4 whereas in PDD, diffuse plaques are common and more prevalent.32 It is possible that differences in the prevalence of neuritic and diffuse plaques may contribute to the differential PiB retention of DLB and PDD. In this regard, a recent study reported significant binding of PiB to both neuritic and diffuse plaques.33 Cerebral amyloid angiopathy (CAA) is also imaged with PiB-PET.22,26 Because varying severity of CAA has been described in both DLB and PDD,34,35 CAA is unlikely to underlie the differential PiB binding of DLB and PDD. Because PiB only negligibly stains NFTs36 and Lewy bodies37 at the concentrations used in PET imaging, it is unlikely that differences in PiB retention across these groups reflect differences in NFTs or Lewy bodies.
We assessed regional amyloid deposition across groups and found that after adjusting for global amyloid levels, the occipital region was preferentially spared in the AD group compared with the DLB, PDD, PD, and NC groups. A relation between PiB retention and temporal parietal hypometabolism has been described in AD.38 FDG-PET studies in DLB, PDD, and PD often show the AD pattern of bilateral temporal–parietal hypometabolism, with the additional reduction of metabolism in the occipital cortex.39 It is therefore possible that occipital hypometabolism in these Lewy body diseases is a consequence of this relative occipital amyloid burden. It is not clear why NC subjects also show this finding. Interestingly, PiB imaging in CAA also demonstrates relative occipital retention in comparison with AD.22
We found a significant correlation between levels of cortical PiB retention and cognitive impairment in the PD, PDD, and DLB groups, as measured by the MMSE. We also found that in the pooled DLB, PDD, and PD cohort, amyloid deposition in the parietal (lateral and precuneus)/posterior cingulate region was associated with visuospatial impairments. This relationship was specific for Lewy body diseases, in which visual spatial dysfunction is well described,1 and was not seen in the AD or NC groups. These data raise the possibility that in Lewy body diseases, amyloid deposits directly impair processing of visual information in the parietal lobe. Although we were unable to find other hypothesized relationships between cognitive test performance and regional amyloid burden, we cannot exclude the possibility that sample size limitations may have obscured weaker correlations. Future work with larger sample sizes will be important. Although the groups were imperfectly matched for gender ratio and age, our method of analysis statistically adjusted for both gender imbalances and age differences. The fact that results showed neither gender/main interaction effects nor age effects indicates that these adjustments did not strongly affect results in any case. It is important to note that diagnostic groups were defined according to clinical criteria, rather than neuropathologic criteria. Given the high prevalence of Lewy bodies detected in AD brains, it is possible that some subjects with clinical AD may have additional evidence of Lewy body pathology on neuropathologic evaluation. A further limitation of this study is the lack of structural MRI data that would allow us to adjust for volume loss. This could cause us to underestimate the amount of amyloid burden in subjects with atrophy.
We also observed that striatal amyloid deposition in the DLB and PDD groups was associated with reduced motor impairment. This result suggests that striatal amyloid is not a marker of motor dysfunction in Lewy body diseases and may in fact indicate preservation of motor function in PDD and DLB. Diffuse plaques are common in the striatum in DLB and PDD,40 but to the best of our knowledge, the relationship to motor features in these diseases has not been explored. It is notable that striatal PiB retention is common in sporadic AD and is marked early in the course of familial AD associated with the presenilin I mutation,41 diseases that are not associated with parkinsonism.
AUTHOR CONTRIBUTIONS
J.J.L. conducted the statistical analysis.
Supplementary Material
Address correspondence and reprint requests to Dr. Keith A. Johnson, Division of Nuclear Medicine and Molecular Imaging, 55 Fruit St., Boston, MA 02114; Massachusetts General Hospital, White 427, Boston, MA 02114 kajohnson@partners.org
Supplemental data at www.neurology.org
Supported by grants from the Alzheimer’s Disease Research Center (ADRC) and Harvard Neurodiscovery Center (S.N.G., K.A.J.), Udall PD Center (P50-NS38372; J.H.G.), ADRC (P50-AG05134; J.H.G., K.A.J.), R21-NS060310 (S.N.G., J.H.G., K.A.J.), the Alzheimer Association (IIRG-06-26331; K.A.J.), R01 AG018402 (W.E.K., C.A.M.), R37 AG025516 (W.E.K., C.A.M.), P50 AG05133 (W.E.K., C.A.M.), 3R01AG027435-02S1 (K.A.J.), and P01 AG025204 (W.E.K., C.A.M.).
Disclosure: W.K. and C.A.M.: GE Healthcare holds a license agreement with the University of Pittsburgh based on the PiB technology described in this article. W.K. and C.A.M. are coinventors of PiB and, as such, have a financial interest in this license agreement. GE Healthcare provided no grant support for this study and had no role in the design or interpretation of results or preparation of this article. S.N.G., D.M.R., E.M., J.A.B., J.J.L., D.R.E., T.S., A.J.F., B.T.H., J.H.G., and K.A.J. have no disclosures.
Received February 21, 2008. Accepted in final form June 12, 2008.
REFERENCES
- 1.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 2007;22:1689–1707. [DOI] [PubMed] [Google Scholar]
- 2.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 3.Lippa CF, Duda JE, Grossman M, et al. DLB/PDD Working Group. DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology 2007;68:812–819. [DOI] [PubMed] [Google Scholar]
- 4.Harding AJ, Halliday GM. Cortical Lewy body pathology in the diagnosis of dementia. Acta Neuropathol 2001;102:355–363. [DOI] [PubMed] [Google Scholar]
- 5.Tiraboschi P, Hansen LA, Alford M, et al. Cholinergic dysfunction in diseases with Lewy bodies. Neurology 2000;54:407–411. [DOI] [PubMed] [Google Scholar]
- 6.Mattila PM, Röyttä M, Torikka H, Dickson DW, Rinne JO. Cortical Lewy bodies and Alzheimer-type changes in patients with Parkinson’s disease. Acta Neuropathol 1998;95:576–582. [DOI] [PubMed] [Google Scholar]
- 7.McKeith IG, Burn D. Spectrum of Parkinson’s disease, Parkinson’s dementia, and Lewy body dementia. In: DeKosky ST, ed. Neurologic Clinics. Philadelphia: WB Saunders, 2000:865–883. [DOI] [PubMed] [Google Scholar]
- 8.Aarsland D, Ballard CG, Halliday G. Are Parkinson’s disease with dementia and dementia with Lewy bodies the same entity? J Geriatr Psychiatry Neurol 2004;17:137–145. [DOI] [PubMed] [Google Scholar]
- 9.Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson’s disease. Arch Neurol 1993;50:140–148. [DOI] [PubMed] [Google Scholar]
- 10.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry 1982;140:566–572. [DOI] [PubMed] [Google Scholar]
- 11.McKhann G, Drachman DA, Folstein M, Katzman R, Price DL, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 12.Lang A, Fahn S. Assessment of Parkinson’s disease. In: Munstat TL, ed. Quantification of Neurologic Deficit. Boston: Butterworths, 1989:285–309. [Google Scholar]
- 13.Ferman TJ, Smith GE, Boeve BF, et al. DLB fluctuations: specific features that reliably differentiate DLB from AD and normal aging. Neurology 2004;62:181–187. [DOI] [PubMed] [Google Scholar]
- 14.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308–2314. [DOI] [PubMed] [Google Scholar]
- 15.Grober E, Buschke H, Crystal H, et al. Screening for dementia by memory testing. Neurology 1988;38:900–903. [DOI] [PubMed] [Google Scholar]
- 16.Mathis CA, Wang Y, Holt DP, et al. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem 2003;46:2740–2754. [DOI] [PubMed] [Google Scholar]
- 17.Logan J, Fowler JS, Volkow ND, et al. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 1996;16:834–840. [DOI] [PubMed] [Google Scholar]
- 18.Lopresti BJ, Klunk WE, Mathis CA, et al. Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med 2005;46:1959–1972. [PubMed] [Google Scholar]
- 19.Mintun MA, Larossa GN, Sheline YI, et al. [11C]PiB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 2006;67:446–452. [DOI] [PubMed] [Google Scholar]
- 20.Archer HA, Edison P, Brooks DJ, et al. Amyloid load and cerebral atrophy in Alzheimer’s disease: an (11)C-PiB positron emission tomography study. Ann Neurol 2006;60:145–147. [DOI] [PubMed] [Google Scholar]
- 21.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann Neurol 2006;59:512–519. [DOI] [PubMed] [Google Scholar]
- 22.Johnson KA, Gregas M, Becker JA, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol 2007;62:229–234. [DOI] [PubMed] [Google Scholar]
- 23.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002;15:273–289. [DOI] [PubMed] [Google Scholar]
- 24.Friston KJ, Holmes AP, Worsley KJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 1995;2:189–210. [Google Scholar]
- 25.Rowe CC, Ng S, Ackermann U, et al. Imaging beta-amyloid burden in aging and dementia. Neurology 2007;68:1718–1725. [DOI] [PubMed] [Google Scholar]
- 26.Bacskai BJ, Frosch MP, Freeman SH, et al. Molecular imaging with Pittsburgh Compound B confirmed at autopsy: a case report. Arch Neurol 2007;64:431–434. [DOI] [PubMed] [Google Scholar]
- 27.Maetzler W, Reimold M, Liepelt I, et al. [11C]PIB binding in Parkinson’s disease dementia. Neuroimage 2008;39:1027–1033. [DOI] [PubMed] [Google Scholar]
- 28.Pike KE, Savage G, Villemagne VL, et al. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain 2007;130:2837–2844. [DOI] [PubMed] [Google Scholar]
- 29.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol 1999;45:358–368. [DOI] [PubMed] [Google Scholar]
- 30.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 2006;66:1837–1844. [DOI] [PubMed] [Google Scholar]
- 31.Kemppainen NM, Aalto S, Karrasch M, et al. Cognitive reserve hypothesis: Pittsburgh Compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer’s disease. Ann Neurol 2008;63:112–118. [DOI] [PubMed] [Google Scholar]
- 32.Braak H, Braak E. Cognitive impairment in Parkinson’s disease: amyloid plaques, neurofibrillary tangles, and neuropil threads in the cerebral cortex. J Neural Transm Park Dis Dement Sect 1990;2:45–57. [DOI] [PubMed] [Google Scholar]
- 33.Lockhart A, Lamb JR, Osredkar T, et al. PiB is a non-specific imaging marker of amyloid-beta (Aβ) peptide-related cerebral amyloidosis. Brain 2007;130:2607–2615. [DOI] [PubMed] [Google Scholar]
- 34.Jellinger KA. Prevalence of vascular lesions in dementia with Lewy bodies: a postmortem study. J Neural Transm 2003;110:771–778. [DOI] [PubMed] [Google Scholar]
- 35.Mastaglia FL, Johnsen RD, Byrnes ML, Kakulas BA. Prevalence of amyloid-beta deposition in the cerebral cortex in Parkinson’s disease. Mov Disord 2003;18:81–86. [DOI] [PubMed] [Google Scholar]
- 36.Klunk WE, Wang Y, Huang GF, et al. The binding of 2-(4′-methylaminophenyl)benzothiazole to postmortem brain homogenates is dominated by the amyloid component. J Neurosci 2003;23:2086–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fodero-Tavoletti MT, Smith DP, McLean CA, et al. In vitro characterization of Pittsburgh Compound-B binding to Lewy bodies. J Neurosci 2007;27:10365–10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edison P, Archer HA, Hinz R, et al. Amyloid, hypometabolism, and cognition in Alzheimer disease: an [11C]PiB and [18F]FDG PET study. Neurology 2007;68:501–508. [DOI] [PubMed] [Google Scholar]
- 39.Yong SW, Yoon JK, An YS, Lee PH. A comparison of cerebral glucose metabolism in Parkinson’s disease, Parkinson’s disease dementia and dementia with Lewy bodies. Eur J Neurol 2007;14:1357–1362. [DOI] [PubMed] [Google Scholar]
- 40.Jellinger KA, Attems J. Does striatal pathology distinguish Parkinson disease with dementia and dementia with Lewy bodies? Acta Neuropathol 2006;112:253–260. [DOI] [PubMed] [Google Scholar]
- 41.Klunk WE, Price JC, Mathis CA, et al. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J Neurosci 2007;27:6174–6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.