Abstract
Spontaneous neoplastic transformation develops within days in the NIH 3T3 line of cells through differential inhibition of their proliferation under contact inhibition. A small fraction of the population continues to multiply after saturation density is reached and is selected to progressively increase saturation density in successive rounds of confluence. The degree of selection at confluence depends on the extent of proliferation of some cells in a heterogeneous population. The development of transformed foci is an extension of the same selective process that increases saturation density. The expression of the foci is enhanced with increases in the saturation density of the surrounding cells. Transformation is also induced by moderately reducing the concentration of calf serum in the medium during low-density passages, which allows selection of cells that require less growth factor. Further stepwise reductions in serum increase the degree of transformation. Contact inhibition and reduction in serum concentration select for the same phenotype of cell that increases saturation density and generates transformed foci. There is mounting evidence that selection is a major factor in the development of common epithelial tumors of humans, but it extends over decades rather than days, and the in vivo microenvironment selects from more stable populations of cells than those in culture. The many progressive levels of increased saturation density and transformed focus formation suggest that a very large number of genes participate in neoplastic development. The operational model of variation and selection presented here may aid in understanding chemical carcinogenesis and cancer recurrence after chemotherapy.
Keywords: carcinogenesis, epigenetics, microenvironment, progression, promotion
The continuous proliferation and repeated passage of rodent cells in monolayer culture eventually lead to their neoplastic transformation and capacity to produce tumors when injected into rodents of the same genetic background (1). This alteration was called spontaneous transformation, because it occurred in the absence of any known carcinogenic treatment and was assumed to reflect the spontaneous occurrence of mutations. The probability of appearance in mouse fibroblasts of early aspects of transformation, such as increased saturation density, rose with the concentration of cells used in their repeated passage (2). Passages of newly explanted mouse fibroblasts at densities that minimized cell-cell contact did not increase their saturation density over 200 generations in 11 months of subculturing, nor did they become tumorigenic (3). High-density passages with extensive contact among cells, however, increased their saturation density, and they became tumorigenic within 30 generations in 3 months of passage. The latent period of tumor appearance after injection of cells into mice decreased with increases in their saturation density, indicating a close relationship between the two properties. Inhibiting the proliferation of the BALB/c 3T3 line of mouse fibroblasts by suspension in agar resulted in their transformation on several occasions, whereas subculturing the cells at low population density in exponential proliferation while attached to a solid surface produced no transformation in the same period (4). The maintenance of a diploid line of rat hepatocytes under the selective condition of confluence was a more efficient inducer of tumorigenic capacity than proliferation at lower densities, even when a strong mutagenic carcinogen was added to the latter (5). These findings indicated that selection plays a major role in the spontaneous neoplastic transformation of cells in culture.
The NIH 3T3 established line of mouse fibroblasts expressed very small lightly stained focal areas of overgrowth if maintained at confluence for ≈10 days in 10% calf serum (CS); much larger fully transformed foci appeared in a second round of confluence (6). Cells from such foci were highly tumorigenic in nude mice (7). It was possible to maintain uniformity in a confluent sheet of cells in the first and even in a second round of confluence when CS concentration was reduced to 2%. With very rare exceptions, the cells could be maintained without undergoing visible transformation, as measured by focus formation in 2% CS if they were kept in frequent low-density passages (LDP) in 10% CS. These methodological developments facilitated systematic and quantitative study of the progressive development of spontaneous transformation. The results with NIH 3T3 cells were scattered through the literature over a 10-year period and embedded in data on other related issues, which led to assorted conclusions about the nature of spontaneous transformation. The cardinal results for understanding spontaneous transformation are presented here and unified under an operational mechanism of progressive selection of cells with increasing capacity to proliferate under growth-regulatory conditions.
Materials, Methods, and Results
Significance of Conditions for Assaying and Promoting Spontaneous Transformation. The cells used in these experiments were from a frozen stock of the NIH 3T3 line provided in 1987 by S. A. Aaronson of the National Cancer Institute, Bethesda (8). The specific stock of cells used in the present experiment was described previously (9). The cells, which had been maintained in weekly LDP, were seeded in five cultures per experimental condition in primary (1°) assays for saturation density and transformed foci at 105 per 60-mm dish in 2% or 10% CS for 2 or 3 wk and were designated as follows: [2-2], 2% CS for 2 wk; [2-3], 2% CS for 3 wk; [10-2], 10% CS for 2 wk; and [10-3], 10% CS for 3 wk. For each group, one of the five cultures was fixed in Bouin's fluid and stained with Giemsa at the end of the 1° assay, and the other four were trypsinized for cell counting. Each counted culture was then reseeded in duplicate for secondary (2°) assays, all of which were carried out in 2% CS for 2 wk regardless of the conditions of the 1° assay. That resulted in four lineages per group. Half of the cultures were fixed and stained at 2 wk. The other four were used for tertiary (3°) and quaternary (4°) assays in succession, all under the same conditions as the 2° assay.
The aim of the experiment was to determine the extent to which the different conditions of the 1° assay influenced the saturation densities and transformed focus formation of the succeeding assays under a single common condition. Four independent lineages were maintained in each group to evaluate the variation within the group in the two properties. The experiment was originally intended to discriminate between epigenetic and genetic origins of changes in saturation density and in transformed focus formation. It was assumed that the uniformity of change in these properties among cultures of the same group would favor an epigenetic origin, whereas variation would favor a genetic origin.
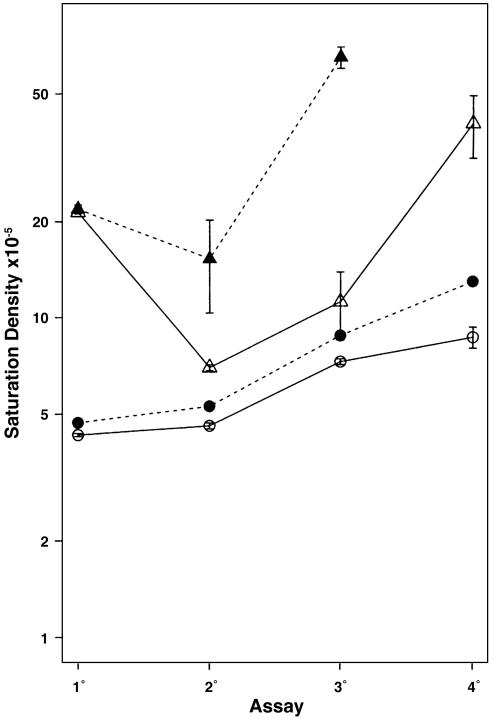
The saturation densities are presented in Fig. 1, and the morphological appearance of the cultures can be seen in Figs. 2 and 3. The saturation densities of [2-3] cultures in the 1° assay were only slightly higher than those in [2-2], indicating very little net proliferation of the cells in the extra week of incubation in 2% CS. The saturation densities in [10-2] and [10-3] for the 1° assay were not significantly different from each other, indicating almost no net proliferation between 2 and 3 wk. The saturation densities of the 1° assays in 10% CS were 5-fold higher than those in 2% CS, signifying a direct relationship with the growth factors in serum.
Fig. 1.
Increases in saturation densities in serial 2°, 3°, and 4° assays at confluence in 2% CS for 2 wk resulting from variations in CS concentration and elapsed time in a preliminary 1° assay. Those 1° assay variations were: [2-2] 2% CS for 2 wk, O-O; [2-3] 2% CS for 3 wk, •---•; [10-2] 10% CS for 2 wk, Δ-Δ; [10-3] 10% CS for 3 wk, ▴---▴. Where standard deviation bars are not seen, they are smaller than the symbols.
Fig. 2.
Assays (1°) in 2% and 10% CS, fixed and stained at 2 and 3 wk. Note the light featureless monolayers in 2% CS at 2 and 3 wk in contrast to the darker cultures in 10% CS with small areas of spontaneous overgrowth, which increase in size and number between 2 and 3 wk, plus one or two larger denser focal areas that resemble early transformed foci.
Fig. 3.
Assays (2°, 3°, and 4°) for 2 wk in 2% CS of four lineages each for sets [2-2], [2-3], [10-2], and [10-3] of the 1° assay. Lineage 1 of set [10-3] was not carried beyond the 2° assay, because it already had so many foci in the 2° assay that it would certainly have produced confluent transformation in the 3° assay. Lineages 2, 3, and 4 of the same set were not carried beyond the 3° assay for the same reason, but they were also diluted 10-fold to 104 cells in the 3° assay and mixed with 105 cells from the standard LDP stock to form a confluent background for display of individual foci. All other assays were seeded with 105 cells. Note the reduction in size and density of the foci of lineages 2 and 3 when the cells were surrounded by LDP cells that had not previously been at confluence. LN, lineage.
The confluent cell sheets in both 1° assays in 2% CS were light and uniform in appearance, with no grossly visible sign of focus formation (Fig. 2). The cultures in 10% CS were much denser throughout and had many very small focal overgrowths, plus one or two slightly larger and still denser focal overgrowths suggestive of early-stage unequivocally transformed foci. The saturation densities of the serial 2°, 3°, and 4° assays that originated from the 1° in 10% CS were much higher than those that originated from 1° assays in 2% CS despite the presence of the same 2% CS for 2 wk in all of the later assays (Fig. 1). The saturation densities, in addition, were much higher in the [10-3] group than in the [10-2] group, and that difference increased sharply in successive assays. These differences indicated that considerable increase in capacity for growth at high density had occurred during the extra week in 10% CS despite the lack of significant increase in cell number in the 1° assay. The 2°, 3°, and 4° assays of the [2-3] group were modestly higher than those of the [2-2] group, but the difference between the two gradually increased with successive assays, indicating a moderate increase in capacity for growth at high density during the extra week of the 1° assay despite only a marginal increase in cell number.
The lack of variation in saturation densities within groups of most of the later assays derived from the 1° assays in 2% CS, and the 2° assays derived from the [10-2] 1° assay were originally suggestive of an epigenetic origin, particularly because an increase in saturation density in the absence of dense focus formation would necessarily be derived from a large fraction of the population. However, the presence of many small areas of overgrowth in the 1° assay of the [10-2] group and their increase in size and number in the [10-3] group (Fig. 2) introduce the likelihood of selection of cells with an advantage for overgrowth at high density in 10% CS. Although there were no grossly visible focal cell densities in 1° assays of the [2-2] and [2-3] groups, microscopic examination revealed such a trend. Therefore, opportunity for selective growth in 2% CS was present, but its extent was limited because of the low overall saturation density. Selective growth despite the meager net increases in cell number between 2 and 3 wk in both 2% and 10% CS can be explained by an offsetting detachment of cells with low capacity for survival in extended periods at high population density.
There was great variability in saturation density among the 2° assays of the [10-3] group, which could be credited to the appearance of many large, dense, transformed foci in lineage 1 and lesser but variable numbers in the other lineages within the group (Fig. 3). Similarly, large and variable numbers of foci appeared in the 3° and 4° assays of the [10-2] group, which accounted for the variability of saturation densities in those assays. The extra week of the [10-3] group in the 1° assay gave it a considerable head start over the [10-2] group in transformed focus formation as well as saturation density. Significant numbers of transformed foci did not appear until the 4° assay of the groups originating from 1° assays in 2% CS, and they were much smaller than those originating from 1° assays in 10% CS. The foci were larger and more common among the [2-3] than the [2-2] lineages, again indicating that the extra week in the 1° assay allowed significant progress in transformation despite only marginal net increase in cell number.
Because lineage 1 of the 2° assay of the [10-3] group exhibited many dense foci (Fig. 3), it was terminated at that point. Based on the presence of some dense foci in the 2° assay of the other lineages of the [10-3] group, it was anticipated that a straight 3° assay of 105 cells might give too many overlapping foci to discern their individual morphology. Therefore, 104 cells of the 2° assay were mixed with 105 LDP stock cells to make a uniform monolayered background against which discrete foci could be displayed. The foci of lineages 2 and 3 from the 105 cell 3° assay were larger and denser than those from the 104 cell assay, whereas light foci from the 105 cell assay of lineage 2 disappeared entirely in the 104 cell assay. This difference in focus formation indicated that the nonfocus-forming background of the straight assay of 105 cells, which consisted of cells that had undergone the same two rounds of confluence as their sibling focus formers, was more permissive to the development of foci than were the LDP stock cells that formed the background for the 104 cell assay.
The promoting effect of increased saturation density on the development of transformed foci suggested that the development of dense foci might preferentially occur within broad light foci if the latter contained enough cells to constitute a significant fraction of the entire population. In Fig. 4, such foci occupy a large fraction of the surface of a culture at 14 days, and some of them display the punctate beginnings of dense foci (10). Given another 7 days to develop, these foci within foci have become more prominent and, in several cases, they are coalescing to form large dense foci, whereas no such changes appear in the flat background that surrounds the light foci. These observations visually confirm that continued proliferation at confluence engenders progressive neoplastic development. If it is assumed that the light foci contain no more than 1,000 cells, and at least 10 dense focal areas arise within each light focus, then the rate of progression of this step is 10-2 or higher per cell division.
Fig. 4.
Origin of dense foci within light foci. The original 1987 frozen stock of NIH 3T3 cells was thawed and cultured for 3 days in 10% CS before subculture of 105 cells for the 1° assay in 2% CS. The culture was fixed and stained at 14 days (Left) and at 21 days (Right). Broad very light foci are visible in the 14-day culture, a few of them containing tiny groups of densely stained cells. The light foci have become slightly darker in the 21-day culture and contain multiple darkly stained punctate foci that in some cases are coalescing to form large dense foci.
Transformation in LDP with Low CS. Variation of transformed focus formation among cultures within groups, which included their size, density, and number as well as the time of their appearance (Fig. 3), suggested they might arise by the induction of new mutations rather than by progressive selection of spontaneous variants that accounted for the increases in saturation density (9). That suggestion was reinforced by the absence of transformed foci in three of the four groups until the 3° or 4° assay and by the later observation that all of the cells recovered after long-term confluence had heritable reductions in rate of proliferation in LDP (11, 12), indicative of chromosomal damage that might transform a small fraction of them. However, increases in saturation density occurred in LDP (2-5 × 104 cells three times per wk in 60-mm dishes) when the CS concentration was reduced from 10% to 2% and was more efficient than with prolonged confluence in 2% (Fig. 5) (13). In parallel with the increases in saturation density, transformed foci appeared in 1° assays made from the LDP in 2% CS (Fig. 6) (14). The cells proliferated exponentially in the LDP, albeit at a 20% lower rate than in 10% CS (15), with no indication of heritable damage (16). Further stepwise reduction of CS in LDP to 1% resulted in an increase in the size and density of the foci, which was more apparent when the assays were done in 1% rather than 2% CS (Fig. 7). A single large step-down from 10% to 1% CS in LDP was much less effective in producing transformed foci than an intermediate multipassage step through 2% CS to 1% CS. Multistep reduction to 0.25% CS followed by many passages in 0.25% CS resulted in exponential proliferation and high saturation density with transformation at that very low CS concentration, which ordinarily supports no detectable proliferation in the absence of selection. These results indicate that the production of foci occurs by the same process as the increases in saturation density but involves further selection of heritable variants that are continuously generated in cultures.
Fig. 5.
Rates of increase in saturation density during LDP in 2% CS vs. undisturbed maintenance in 2% CS. Groups of cultures were maintained in LDP in 10% CS (A), LDP in 2% CS (B), and undisturbed maintenance in 2% CS (C). At the intervals in days indicated at the curves in B, the cells in A and B were serially subcultured at low density. On the same days, as indicated in C, two cultures were trypsinized. At each interval, 105 cells from each group were seeded in 2% CS and counted for their saturation density at 2 wk.
Fig. 6.
Appearance of transformed foci in 1° assay initiated by cells after each LDP in 2% CS. The cells were the same as those used in Fig. 5B, in which LDP of cells were made at 2, 4, 7, 9, 11, and 14 days, and 105 cells of each passage were seeded in 2% CS for 2 wk. The photograph shows sister cultures to those in Fig. 5B, but they are fixed and stained to display transformed foci rather than trypsinized and counted for saturation densities. [Reproduced with permission from ref. 14 (Copyright 1990, American Association for Cancer Research).]
Fig. 7.
Transformed foci made by cells that had undergone 12 successive LDP in 10% or 2% CS or six passages in 10% and six passages in 1% CS or six passages in 2% and six passages in 1% CS, as shown. Cells (105) from each category were assayed in a 1° assay in 2% and 1% CS for 14 days, then fixed and stained. [Reproduced with permission from ref. 14 (Copyright 1990, American Association for Cancer Research).]
Conclusions
The degree of neoplastic transformation is measured here by increases in saturation density of NIH 3T3 cell cultures and the appearance of transformed foci in various sizes, densities, and numbers in standardized serial assays at confluence.
The degree of transformation in serial assays at confluence depends on the extent of proliferation of selected cells under contact inhibition. The extent of proliferation at confluence in a 1° assay is determined by the concentration of CS in the medium and the time interval spent under contact inhibition. Progressive increases in saturation density and focus formation in the subsequent serial assays, all under a single standardized condition, depend on the extent of transformation in the prior assays. This compounding of increases in transformation arises because each of the successive assays reaches a higher saturation density, allowing more proliferation and therefore more selection to occur under contact inhibition.
The higher the saturation density of the cultures the more permissive it is for the expression of foci initiated by those cells that have progressed the furthest in their transformed state. A background of cells that have not been selected for transformation tends to suppress focus formation by cells from a culture selected for growth under inhibitory conditions. One subline that underwent >100 LDP in high serum concentration and was then seeded in large numbers together with a small number of transformed cells allowed the appearance of small foci until the former underwent contact inhibition, when they gradually obliterated the foci (17). This illustrates that importance of the cellular microenvironment in tumor development (18).
Increases of saturation density and transformed focus formation are two sides of the same coin of transformation. Both represent selection for capacity to proliferate at high population density, but increased saturation density requires the contemporaneous selection of many cells or the presence of large dense foci that add significantly to the total number of cells in the population. The transformed foci themselves originate from single cells that constitute a small minority, more advanced in transformation than the surrounding cells.
Transformation can also be brought about during LDP by lowering the concentration of CS in the medium to a level that moderately decreases the rate of cell proliferation, thereby allowing the selection of cells better able to proliferate under the restricted condition. Too steep a step-down in CS concentration sharply decreases proliferation of the selectable cells and inhibits progression. A stepwise decrease in CS concentrations that allows moderate proliferation at each step, selectively increases the degree of transformation. Combining a moderately inhibitory CS concentration with contact inhibition acts like too steep a step-down in CS by decreasing proliferation of both selectable and nonselectable cells, thereby restraining progression. Combining high CS with LDP maximizes proliferation of selectable and nonselectable cells to a similar extent and minimizes progression.
Contact inhibition, reduction in serum concentration, and suspension in agar appear to inhibit proliferation by reducing the activity of the cell membrane. They progressively select for cells with increasing membrane activity under inhibitory conditions, which characterizes increasing degrees of transformation. Thus, the production of transformed foci, proliferation in low serum concentrations, and colony formation in agar are differential expressions of the same cellular phenotype. Tumorigenesis apparently sets a higher and perhaps different bar to proliferation, because the most fully transformed cells derived in cell culture require much more time to produce detectable tumors (18-33 days) after injection into nude mice than do cells derived from those tumors (5 days) after one passage in culture and reinjection into mice (7). In addition, cells explanted from delayed tumors that are adapted to proliferate in nude mice are greatly diminished in their capacity to do so in culture (19), indicating major differences in the growth-promoting milieu in vivo and in vitro.
There is increasing evidence that selection plays a major role in the development of human cancers of the lung (20), colon (21), and skin (22). It is also considered the mechanism of promotion in the classical two-stage model of skin carcinogenesis in mice (23). Hyperplasia, which occurs at an early stage of cancer of the lung (24), colon (25), and skin (26), may offer a parallel to increased saturation density in providing a permissive selective milieu for neoplastic progression. The rate of selectable physiological and genetic changes is much lower and better regulated in the organism than in cell culture and therefore stretches out the selective process for cancer over decades rather than the few days required for transformation under imposed selection in the NIH 3T3 cells. However, the latter has allowed efficient systematic study of the dynamics of the process. Other studies made with diploid rat liver epithelial cells showed that selection of spontaneous variants is far more effective in generating liver cancer cells than is treatment with a powerful mutagenic carcinogen under minimally selective conditions (5). The relative contributions of mutation and selection to neoplastic development may be reversed in the animal, where the cells in their tissue microenvironment are much more stable than in culture. It should be noted, however, that carcinogens can promote selection in animals by disrupting the microenvironment, in addition to increasing the frequency of genetic change (22).
The gradual increase in saturation density with repeated rounds of confluence in groups [2-2] and [2-3] of Fig. 1, and in LDP with low CS in Fig. 5B, suggests that a very large number of genetic changes can individually produce small additive increases in the neoplastic behavior of cells. A similar suggestion arises from the many progressive changes in size and density of transformed foci, most apparent in Fig. 3. The most common genetic changes in human epithelial tumors are allelic deletions (27), which exceed one-third of the informative loci in many colon cancers (28). These multilocus deletions rearrange the order of genes at the rejoined chromosomal ends. The partial loss of activity among tumor suppressor genes or complete loss of some of them through mutation of the remaining allele could account for increments in neoplastic behavior of cells under selection, as described here. The requirement that at least 80% of cells in tumors must carry a particular deletion for it to be chemically detectable (28) is consistent with a selective role of such deletions in neoplastic development.
The conclusions presented here were generated through operational analysis under defined conditions (29) in which only the observed behavior of living cells is considered, and only limited inference is made about molecular mechanisms, because of their complexity. An indication of the complexity of genetic analysis comes from the observations that (i) every cell of the NIH 3T3 line examined by chromosome banding exhibits a unique karyotype that is continuously changing (30), and (ii) there is enormously wide variation in focus formation of clones and subclones from the same population (13). Any attempt to fully trace causal chains of transformation in such a system is inevitably lost in its immense complexity (31). In contrast, operational analysis of observable cell behavior yields a model of transformation that unifies seemingly disparate methods of selection and expression of incrementally transformed states.
The operational model presented here should be applicable in principle to the mechanism of carcinogenesis in the organism. Chemical carcinogenesis of the liver (32) or skin (33) in experimental animals and UV carcinogenesis of the skin in humans (22) select for cells resistant to the causative agents. The selective basis for neoplastic development has been generalized to many other human cancers (34). Recurrence of cancer after chemotherapy may follow the extremely heterogeneous pattern of cellular resistance to bromodeoxyuridine established over prolonged exposure in cultured cells (35), which is similar to the evolution of cells described above that escape physiological regulation to generate spontaneous transformation.
Acknowledgments
I am grateful to Dorothy M. Rubin for manuscript preparation and editing. Drs. Ming Chow, John Gerhart, and Steven Martin made valuable comments. The effort for this paper was supported by National Institutes of Health Grant G13LM07483-03.
Abbreviations: CS, calf serum; LDP, low-density passages; 1°, primary assay; 2°, secondary assay; 3°, tertiary assay; 4°, quaternary assay.
References
- 1.Sanford, K. K. & Evans, V. J. (1982) J. Natl. Cancer Inst. 68, 895-913. [PubMed] [Google Scholar]
- 2.Todaro, G. J. & Green, H. (1963) J. Cell Biol. 17, 299-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aaronson, S. A. & Todaro, G. J. (1968) Science 162, 1024-1026. [DOI] [PubMed] [Google Scholar]
- 4.Rubin, H. (1988) Cancer Res. 48, 2512-2518. [PubMed] [Google Scholar]
- 5.Lee, L. W., Tsao, M.-S., Grisham, J. W. & Smith, G. J. (1989) Am. J. Pathol. 135, 63-71. [PMC free article] [PubMed] [Google Scholar]
- 6.Rubin, H. & Xu, K. (1989) Proc. Natl. Acad. Sci. USA 86, 1860-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubin, A. L., Arnstein, P. & Rubin, H. (1990) Proc. Natl. Acad. Sci. USA 87, 10005-10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jainchill, J. L., Aaronson, S. A. & Todaro, G. J. (1969) J. Virol. 4, 549-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubin, H. (1994) Proc. Natl. Acad. Sci. USA 91, 12076-12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow, M. & Rubin, H. (1999) Proc. Natl. Acad. Sci. USA 96, 6976-6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubin, H., Yao, A. & Chow, M. (1995) Proc. Natl. Acad. Sci. USA 92, 4843-4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubin, H., Chow, M. & Yao, A. (1996) Proc. Natl. Acad. Sci. USA 93, 1825-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubin, A. L., Yao, A. & Rubin, H. (1990) Proc. Natl. Acad. Sci. USA 87, 482-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao, A., Rubin, A. L. & Rubin, H. (1990) Cancer Res. 50, 5171-5176. [PubMed] [Google Scholar]
- 15.Yao, A. & Rubin, H. (1992) Proc. Natl. Acad. Sci. USA 89, 7486-7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao, A., Huang, W. & Rubin, H. (1991) Proc. Natl. Acad. Sci. USA 88, 9422-9425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubin, H. (1994) Proc. Natl. Acad. Sci. USA 91, 1039-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubin, H. (2003) Adv. Cancer Res. 90, 1-62. [DOI] [PubMed] [Google Scholar]
- 19.Rubin, H., Chu, B. M. & Arnstein, P. (1987) Cancer Res. 47, 486-492. [PubMed] [Google Scholar]
- 20.Rodin, S. N. & Rodin, A. S. (2000) Proc. Natl. Acad. Sci. USA 97, 12244-12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomlinson, I. & Bodmer, W. (1999) Nat. Med. 5, 11-12. [DOI] [PubMed] [Google Scholar]
- 22.Zhang, W., Remenyik, E., Zelterman, D., Brash, D. E. & Wikonkai, N. M. (2001) Proc. Natl. Acad. Sci. USA 98, 13948-13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuspa, S. H., Ben, T., Hennings, H. & Lichti, U. (1982) Cancer Res. 42, 2344-2349. [PubMed] [Google Scholar]
- 24.Auerbach, O., Stout, A. P., Hammond, E. C. & Garfinkel, L. (1962) N. Engl. J. Med. 267, 119-125. [DOI] [PubMed] [Google Scholar]
- 25.Chang, W. (1978) J. Natl. Cancer Inst. 60, 1405-1418. [DOI] [PubMed] [Google Scholar]
- 26.Setala, K., Merenmies, L., Stjernvall, L., Aho, Y. & Kajanne, P. (1959) J. Natl. Cancer Inst. 23, 925-951. [PubMed] [Google Scholar]
- 27.Rodriguez, E., Sreekanitalah, C. & Chaganti, R. S. K. (1994) Cancer Res. 54, 3398-3406. [PubMed] [Google Scholar]
- 28.Vogelstein, B., Fearon, E. C., Kern, S. E., Hamilton, S. R., Preisinger, A. C., Nakamura, Y. & White, R. (1989) Science 244, 207-211. [DOI] [PubMed] [Google Scholar]
- 29.Bridgman, P. W. (1958) Reflections of a Physicist (Philosophical Library, New York).
- 30.Rubin, H. (1993) Differentiation (Berlin) 53, 123-137. [DOI] [PubMed] [Google Scholar]
- 31.Elsasser, W. M. (1998) Reflections on a Theory of Organisms (Johns Hopkins Univ. Press, Baltimore).
- 32.Farber, E. & Sarma, D. S. R. (1987) Lab. Invest. 56, 4-22. [PubMed] [Google Scholar]
- 33.Vasiliev, J. M. & Guelstein, V. I. (1963) J. Natl. Cancer Inst. 31, 1123-1143. [PubMed] [Google Scholar]
- 34.Farber, E. (1984) Cancer Res. 44, 4217-4223. [PubMed] [Google Scholar]
- 35.Harris, M. & Collier, K. (1980) Proc. Natl. Acad. Sci. USA 77, 4206-4210. [DOI] [PMC free article] [PubMed] [Google Scholar]